- Record: found
- Abstract: found
- Article: found
Gastrointestinal Stromal Tumors Associated with Neurofibromatosis 1: A Single Centre Experience and Systematic Review of the Literature Including 252 Cases
Read this article at
Abstract
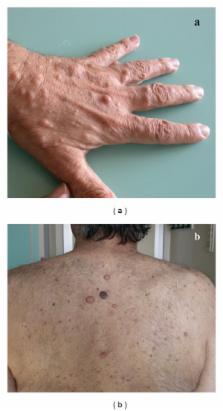
Aims. The objectives of this study were (a) to report our experience regarding the association between neurofibromatosis type 1 (NF1) and gastrointestinal stromal tumors (GISTs); (b) to provide a systematic review of the literature in this field; and (c) to compare the features of NF1-associated GISTs with those reported in sporadic GISTs. Methods. We reported two cases of NF1-associated GISTs. Moreover we reviewed 23 case reports/series including 252 GISTs detected in 126 NF1 patients; the data obtained from different studies were analyzed and compared to those of the sporadic GISTs undergone surgical treatment at our centre. Results. NF1 patients presenting with GISTs had a homogeneous M/F ratio with a mean age of 52.8 years. NF1-associated GISTs were often reported as multiple tumors, mainly incidental, localized at the jejunum, with a mean diameter of 3.8 cm, a mean mitotic count of 3.0/50 HPF, and KIT/PDGFR α wild type. We reported a statistical difference comparing the age and the symptoms at presentation, the tumors' diameters and localizations, and the risk criteria of the NF1-associated GISTs comparing to those documented in sporadic GISTs. Conclusions. NF1-associated GISTs seem to have a distinct phenotype, specifically younger age, distal localization, small diameter, and absence of KIT/PDGRF α mutations.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden.
- Record: found
- Abstract: found
- Article: not found
Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis.
- Record: found
- Abstract: not found
- Article: not found