- Record: found
- Abstract: found
- Article: found
In-vitro Activity of Avermectins against Mycobacterium ulcerans
Read this article at
Abstract
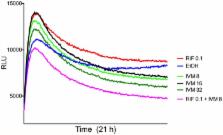
Mycobacterium ulcerans causes Buruli ulcer (BU), a debilitating infection of subcutaneous tissue. There is a WHO-recommended antibiotic treatment requiring an 8-week course of streptomycin and rifampicin. This regime has revolutionized the treatment of BU but there are problems that include reliance on daily streptomycin injections and side effects such as ototoxicity. Trials of all-oral treatments for BU show promise but additional drug combinations that make BU treatment safer and shorter would be welcome. Following on from reports that avermectins have activity against Mycobacterium tuberculosis, we tested the in-vitro efficacy of ivermectin and moxidectin on M. ulcerans. We observed minimum inhibitory concentrations of 4–8 μg/ml and time-kill assays using wild type and bioluminescent M. ulcerans showed a significant dose-dependent reduction in M. ulcerans viability over 8-weeks. A synergistic killing-effect with rifampicin was also observed. Avermectins are well tolerated, widely available and inexpensive. Based on our in vitro findings we suggest that avermectins should be further evaluated for the treatment of BU.
Author Summary
Neglected tropical diseases such as Buruli ulcer predominantly afflict the poorest populations in the world and reduce quality of life. Buruli ulcer is a necrotising infection that destroys the skin and soft tissue, frequently presenting as nodules or open ulcers. Buruli ulcer is treated with antibiotics and sometimes surgery. Unfortunately the antibiotic treatment can have toxic side effects, such as hearing loss. Also, patients must either be hospitalized or report daily to a treatment centre to get their medicine as the treatment is delivered by injection.
In laboratory experiments we tested the susceptibility of Mycobacterium ulcerans, which causes Buruli ulcer, to avermectins. Avermectins are drugs that are used to treat common parasite and worm infections, such as river blindness. These drugs are inexpensive, have few side effects and are widely available. Our findings show that two avermectins called ivermectin and moxidectin inhibit the growth and also kill Mycobacterium ulcerans strains from both Africa and Australia. If their efficacy and safety also can be proven in animal and human studies, these drugs will provide an inexpensive addition to the current treatment of Buruli ulcer.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis.
- Record: found
- Abstract: found
- Article: not found
Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer.
- Record: found
- Abstract: found
- Article: not found