- Record: found
- Abstract: found
- Article: found
Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment
Read this article at
Abstract
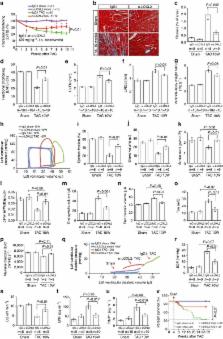
Interstitial fibrosis plays a key role in the development and progression of heart failure. Here, we show that an enzyme that crosslinks collagen—Lysyl oxidase-like 2 (Loxl2)—is essential for interstitial fibrosis and mechanical dysfunction of pathologically stressed hearts. In mice, cardiac stress activates fibroblasts to express and secrete Loxl2 into the interstitium, triggering fibrosis, systolic and diastolic dysfunction of stressed hearts. Antibody-mediated inhibition or genetic disruption of Loxl2 greatly reduces stress-induced cardiac fibrosis and chamber dilatation, improving systolic and diastolic functions. Loxl2 stimulates cardiac fibroblasts through PI3K/AKT to produce TGF-β2, promoting fibroblast-to-myofibroblast transformation; Loxl2 also acts downstream of TGF-β2 to stimulate myofibroblast migration. In diseased human hearts, LOXL2 is upregulated in cardiac interstitium; its levels correlate with collagen crosslinking and cardiac dysfunction. LOXL2 is also elevated in the serum of heart failure (HF) patients, correlating with other HF biomarkers, suggesting a conserved LOXL2-mediated mechanism of human HF.
Abstract
Lysyl oxidase-like 2 (LOXL2) is an enzyme that promotes scaffolding of extracellular matrix proteins. Here the authors show that LOXL2 is crucial for pressure-overload induced cardiac fibrosis, and that antibody-mediated inhibition or genetic disruption of Loxl2 in mice shows therapeutic potential for treatment of cardiac fibrosis.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system.
- Record: found
- Abstract: found
- Article: not found
Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment.

- Record: found
- Abstract: found
- Article: found