- Record: found
- Abstract: found
- Article: found
22‐S‐Hydroxycholesterol protects against ethanol‐induced liver injury by blocking the auto/paracrine activation of MCP‐1 mediated by LXRα
Read this article at
Abstract
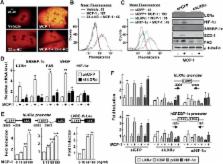
Chronic ethanol consumption causes hepatic steatosis and inflammation, which are associated with liver hypoxia. Monocyte chemoattractant protein‐1 ( MCP‐1) is a hypoxia response factor that determines recruitment and activation of monocytes to the site of tissue injury. The level of MCP‐1 is elevated in the serum and liver of patients with alcoholic liver disease ( ALD); however, the molecular details regarding the regulation of MCP‐1 expression are not yet understood completely. Here, we show the role of liver X receptor α ( LXR α) in the regulation of MCP‐1 expression during the development of ethanol‐induced fatty liver injury, using an antagonist, 22‐ S‐hydroxycholesterol (22‐ S‐ HC). First, administration of 22‐ S‐ HC attenuated the signs of liver injury with decreased levels of MCP‐1 and its receptor CCR2 in ethanol‐fed mice. Second, hypoxic conditions or treatment with the LXR α agonist GW3965 significantly induced the expression of MCP‐1, which was completely blocked by treatment with 22‐ S‐ HC or infection by shLXR α lentivirus in the primary hepatocytes. Third, over‐expression of LXR α or GW3965 treatment increased MCP‐1 promoter activity by increasing the binding of hypoxia‐inducible factor‐1 α to the hypoxia response elements, together with LXR α. Finally, treatment with recombinant MCP‐1 increased the level of expression of LXR α and LXR α‐dependent lipid droplet accumulation in both hepatocytes and Kupffer cells. These data show that LXR α and its ligand‐induced up‐regulation of MCP‐1 and MCP‐1‐induced LXR α‐dependent lipogenesis play a key role in the autocrine and paracrine activation of MCP‐1 in the pathogenesis of alcoholic fatty liver disease, and that this activation may provide a promising new target for ALD therapy.Copyright © 2014 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif.
- Record: found
- Abstract: found
- Article: not found
Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP).
- Record: found
- Abstract: found
- Article: not found