- Record: found
- Abstract: found
- Article: found
The role of DNMT1/hsa-miR-124-3p/BCAT1 pathway in regulating growth and invasion of esophageal squamous cell carcinoma
Read this article at
Abstract
Background
Esophageal squamous cell carcinoma (ESCC) is the major subtype of esophageal cancer with high aggressiveness and poor prognosis. There is an urgent need for understanding the molecular mechanism underlying the development and progression of ESCC.
Methods
ESCC tissues and corresponding non-neoplastic tissues were collected. The expression and function of miR-124-3p and BCAT1 in two cell lines KYSE-150 and Eca109 were determined.
Results
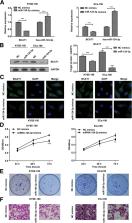
We show downregulation of miR-124-3p expression in ESCC tissues, which is highly correlated with proliferation and migration of ESCC cell lines KYSE-150 and Eca109. miR-124-3p show high correlation with TNM stage and differentiation grade. Furthermore, miR-124-3p directly targets mRNA 3’UTR region of BCAT1, which results in upregulation of BCAT1 expression as observed in ESCC tissues and cell lines. Also, our data indicates that BCAT1 high expression is strongly linked to the disease-free survival, tumor size, pathologic stage, T classification and differentiation grade. On the other hand, we clarified the upstream mechanism regulating miR-124-3p expression in ESCC, which involves in the hypermethylation-silencing regulation mediated by DNA methyltransferase 1(DNMT1), which is of high expression in ESCC tissues and cell lines in the present study. In addition, DNMT1 knockdown or inhibition of DNMT1 function contributes to downregulation of miR-124-3p and BCAT1 expression.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Epigenetic modulators, modifiers and mediators in cancer aetiology and progression.
- Record: found
- Abstract: found
- Article: not found
The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2.
- Record: found
- Abstract: found
- Article: not found