- Record: found
- Abstract: found
- Article: not found
ROX index as a good predictor of high flow nasal cannula failure in COVID-19 patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis
Read this article at
Abstract
Purpose
Prediction of high flow nasal cannula (HFNC) failure in COVID-19 patients with acute hypoxemic respiratory failure (AHRF) may improve clinical management and stratification of patients for optimal treatment. We performed a systematic review and meta-analysis to determine performance of ROX index as a predictor of HFNC failure.
Materials and methods
Systematic search was performed in electronic databases (PubMed, Google Scholar, Web of Science and Cochrane Library) for articles published till 15 June 2021 investigating ROX index as a predictor for HFNC failure. Quality In Prognosis Studies (QUIPS) tool was used to analyze risk of bias for prognostic factors, by two independent authors.
Results
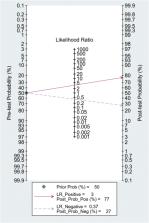
Eight retrospective or prospective cohort studies involving 1301 patients showed a good discriminatory value, summary area under the curve (sAUC) 0.81 (95% CI, 0.77–0.84) with sensitivity of 0.70 (95% CI, 0.59–0.80) and specificity of 0.79 (95% CI, 0.67–0.88) for predicting HNFC failure. The positive and negative likelihood ratio were 3.0 (95% CI, 2.2–5.3) and 0.37 (95% CI, 0.28–0.50) respectively, and was strongly associated with a promising predictive accuracy (Diagnostic odds ratio (DOR) 9, 95% CI, 5–16).
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Quantifying heterogeneity in a meta-analysis.
- Record: found
- Abstract: found
- Article: not found
