- Record: found
- Abstract: found
- Article: found
Carcinoma-Associated Mesenchymal Stem Cells Promote Chemoresistance in Ovarian Cancer Stem Cells via PDGF Signaling
Read this article at
Abstract
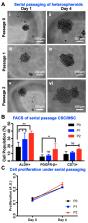
Within the ovarian cancer tumor microenvironment, cancer stem-like cells (CSC) interact with carcinoma associated mesenchymal stem/stromal cells (CA-MSC) through multiple secreted cytokines and growth factors. These paracrine interactions have been revealed to cause enrichment of CSC and their chemoprotection; however, it is still not known if platelet-derived growth factor (PDGF) signaling is involved in facilitating these responses. In order to probe this undiscovered bidirectional communication, we created a model of ovarian malignant ascites in the three-dimensional (3D) hanging drop heterospheroid array, with CSC and CA-MSC. We hypothesized that PDGF secretion by CA-MSC increases self-renewal, migration, epithelial to mesenchymal transition (EMT) and chemoresistance in ovarian CSC. Our results indicate that PDGF signaling in the CSC-MSC heterospheroids significantly increased stemness, metastatic potential and chemoresistance of CSC. Knockdown of PDGFB in MSC resulted in abrogation of these phenotypes in the heterospheroids. Our studies also reveal a cross-talk between PDGF and Hedgehog signaling in ovarian cancer. Overall, our data suggest that when the stromal signaling via PDGF to ovarian CSC is blocked in addition to chemotherapy pressure, the tumor cells are significantly more sensitive to chemotherapy. Our results emphasize the importance of disrupting the signals from the microenvironment to the tumor cells, in order to improve response rates. These findings may lead to the development of combination therapies targeting stromal signaling (such as PDGF and Hedgehog) that can abrogate the tumorigenic, metastatic and platinum resistant phenotypes of ovarian CSC through additional investigations.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer.
- Record: found
- Abstract: found
- Article: not found
Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival.
- Record: found
- Abstract: found
- Article: not found