- Record: found
- Abstract: found
- Article: found
Homeostatic Tissue Responses in Skin Biopsies from NOMID Patients with Constitutive Overproduction of IL-1β
Read this article at
Abstract
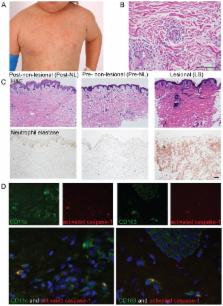
The autoinflammatory disorder, Neonatal- onset Multisystem Inflammatory Disease (NOMID) is the most severe phenotype of disorders caused by mutations in CIAS1 that result in increased production and secretion of active IL-1β. NOMID patients present with systemic and organ-specific inflammation of the skin, central nervous system and bone, and respond dramatically to treatment with IL-1 blocking agents. We compared the cellular infiltrates and transcriptome of skin biopsies from patients with NOMID (n = 14) before treatment (lesional (LS) and non-lesional (pre-NL) skin) and after treatment (post-NL) with the IL-1 blocker anakinra (recombinant IL-1 receptor antagonist, Kineret®, Swedish Orphan Biovitrum AB, SOBI), to normal skin (n = 5) to assess tissue responses in the context of untreated and treated disease. Abundant neutrophils distinguish LS skin from pre-NL and post-NL skin. CD11c + dermal dendritic cells and CD163 + macrophages expressed activated caspase-1 and are a likely source of cutaneous IL-1 production. Treatment with anakinra led to the disappearance of neutrophils, but CD3 + T cells and HLA-DR + cells remained elevated. Among the upregulated genes IL-6, IL-8, TNF, IL-17A, CCL20, and the neutrophil defensins DEFA1 and DEFA3 were differentially regulated in LS tissues (compared to normal skin). Important significantly downregulated pathways in LS skin included IL-1R/TLR signaling, type I and II cytokine receptor signaling, mitochondrial dysfunction, and antigen presentation. The differential expression and regulation of microRNAs and pathways involved in post-transcriptional modification were suggestive of epigenetic modification in the chronically inflamed tissue. Overall, the dysregulated genes and pathways suggest extensive “adaptive” mechanisms to control inflammation and maintain tissue homeostasis, likely triggered by chronic IL-1 release in the skin of patients with NOMID.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis.
- Record: found
- Abstract: found
- Article: not found
A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes.
- Record: found
- Abstract: found
- Article: not found