- Record: found
- Abstract: found
- Article: found
Dual inhibition of CDK12 and CDK13 uncovers actionable vulnerabilities in patient-derived ovarian cancer organoids
Read this article at
Abstract
Background
High grade serous ovarian cancer (HGSOC) is highly lethal, partly due to chemotherapy resistance and limited availability of targeted approaches. Cyclin dependent kinases 12 and 13 (CDK12/13) are promising therapeutic targets in human cancers, including HGSOC. Nevertheless, the effects of their inhibition in HGSOC and the potential synergy with other drugs are poorly known.
Methods
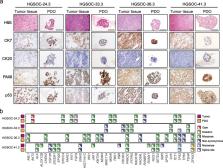
We analyzed the effects of the CDK12/13 inhibitor THZ531 in HGSOC cells and patient-derived organoids (PDOs). RNA sequencing and quantitative PCR analyses were performed to identify the genome-wide effects of short-term CDK12/13 inhibition on the transcriptome of HGSOC cells. Viability assays with HGSOC cells and PDOs were performed to assess the efficacy of THZ531 as single agent or in combination with clinically relevant drugs.
Results
The CDK12 and CDK13 genes are deregulated in HGSOC and their concomitant up-regulation with the oncogene MYC predicts poor prognosis. HGSOC cells and PDOs display high sensitivity to CDK12/13 inhibition, which synergizes with drugs in clinical use for HGSOC. Transcriptome analyses revealed cancer-relevant genes whose expression is repressed by dual CDK12/13 inhibition through impaired splicing. Combined treatment with THZ531 and inhibitors of pathways regulated by these cancer relevant genes ( EGFR, RPTOR, ATRIP) exerted synergic effects on HGSOC PDO viability.
Conclusions
CDK12 and CDK13 represent valuable therapeutic targets for HGSOC. We uncovered a wide spectrum of CDK12/13 targets as potential therapeutic vulnerabilities for HGSOC. Moreover, our study indicates that CDK12/13 inhibition enhances the efficacy of approved drugs that are already in use for HGSOC or other human cancers.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: found
Hallmarks of Cancer: The Next Generation
- Record: found
- Abstract: found
- Article: not found
Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support.
- Record: found
- Abstract: found
- Article: not found