- Record: found
- Abstract: found
- Article: found
Improving antitumor immunity using antiangiogenic agents: Mechanistic insights, current progress, and clinical challenges

Read this article at
Abstract
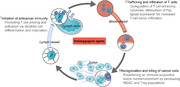
Cancer immunotherapy, especially immune checkpoint blockade (ICB), has revolutionized oncology. However, only a limited number of patients benefit from immunotherapy, and some cancers that initially respond to immunotherapy can ultimately relapse and progress. Thus, some studies have investigated combining immunotherapy with other therapies to overcome resistance to monotherapy. Recently, multiple preclinical and clinical studies have shown that tumor vasculature is a determinant of whether immunotherapy will elicit an antitumor response; thus, vascular targeting may be a promising strategy to improve cancer immunotherapy outcomes. A successful antitumor immune response requires an intact “Cancer‐Immunity Cycle,” including T cell priming and activation, immune cell recruitment, and recognition and killing of cancer cells. Angiogenic inducers, especially vascular endothelial growth factor (VEGF), can interfere with activation, infiltration, and function of T cells, thus breaking the “Cancer‐Immunity Cycle.” Together with immunostimulation‐regulated tumor vessel remodeling, VEGF‐mediated immunosuppression provides a solid therapeutic rationale for combining immunotherapy with antiangiogenic agents to treat solid tumors. Following the successes of recent landmark phase III clinical trials, therapies combining immune checkpoint inhibitors (ICIs) with antiangiogenic agents have become first‐line treatments for multiple solid tumors, whereas the efficacy of such combinations in other solid tumors remains to be validated in ongoing studies. In this review, we discussed synergies between antiangiogenic agents and cancer immunotherapy based on results from preclinical and translational studies. Then, we discussed recent progress in randomized clinical trials. ICI‐containing combinations were the focus of this review because of their recent successes, but combinations containing other immunotherapies were also discussed. Finally, we attempted to define critical challenges in combining ICIs with antiangiogenic agents to promote coordination and stimulate collaboration within the research community.
Abstract
In this review, mechanisms of VEGF‐induced immunosuppression based on results from preclinical and translational studies and recent progress in randomized clinical trials were discussed. Key challenges associated with dual targeting of VEGF and PD‐1/PD‐L1 pathways were also defined in order to stimulate collaboration and help galvanize a broader effort.
Related collections
Most cited references123
- Record: found
- Abstract: found
- Article: found
Hallmarks of Cancer: The Next Generation
- Record: found
- Abstract: found
- Article: not found
Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found