- Record: found
- Abstract: found
- Article: found
The prognostic impact of inflammatory factors in patients with multiple myeloma treated with thalidomide in Korea
Read this article at
Abstract
Background/Aims:
The purpose of this study was to determine the correlations between inflammatory factors—including absolute lymphocyte count, lactate dehydrogenase, β2-microglobulin, albumin, C-reactive protein, and ferritin—and the prognosis for survival in patients with multiple myeloma (MM) treated with induction chemotherapy containing thalidomide and who underwent autologous stem cell transplantation (ASCT).
Methods:
Data from patients at 13 university hospitals in South Korea were collected retrospectively between December 2005 and May 2013.
Results:
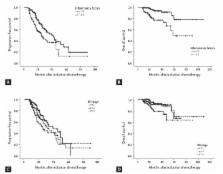
The median age of the 232 patients was 57 years (range, 33 to 77) and the male to female ratio was 1.09:1. In the multivariate analysis, fewer than two combined abnormal inflammatory factors was the only independent prognostic factor for superior progression-free survival (relative risk [RR], 0.618; 95% confidence interval [CI], 0.409 to 0.933; p = 0.022), and platelet count > 100 × 10 9/L and fewer than two combined abnormal inflammatory factors were independent prognostic factors for superior overall survival (RR, 4.739; 95% CI, 1.897 to 11.839; p = 0.001 and RR, 0.263; 95% CI, 0.113 to 0.612; p = 0.002, respectively).
Conclusions:
Patients with two or more than two combined inflammatory factors who were treated with thalidomide induction chemotherapy and who underwent ASCT showed significantly shorter survival compared to those with fewer than two combined inflammatory factors. These results could be helpful for predicting prognosis in patients with MM.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival.
- Record: found
- Abstract: found
- Article: not found
Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study.
- Record: found
- Abstract: found
- Article: not found