- Record: found
- Abstract: found
- Article: found
C-reactive protein and procalcitonin to discriminate between tuberculosis, Pneumocystis jirovecii pneumonia, and bacterial pneumonia in HIV-infected inpatients meeting WHO criteria for seriously ill: a prospective cohort study
Abstract
Background
Tuberculosis, bacterial community-acquired pneumonia (CAP), and Pneumocystis jirovecii pneumonia (PJP) are the three commonest causes of hospitalisation in HIV-infected adults. Prompt diagnosis and treatment initiation are important to reduce morbidity and mortality, but are hampered by limited diagnostic resources in resource poor settings. C-reactive protein (CRP) and procalcitonin have shown diagnostic utility for respiratory tract infections, however few studies have focussed on their ability to distinguish between tuberculosis, CAP, and PJP in HIV-infected inpatients.
Methods
We evaluated the diagnostic accuracy of CRP and procalcitonin, compared with composite reference standards, to discriminate between the three target infections in adult HIV-infected inpatients in two district level hospitals in Cape Town, South Africa. Participants were admitted with current cough and danger signs in accordance with the WHO algorithm for tuberculosis in seriously ill HIV-infected patients. Study clinicians were blinded to CRP and procalcitonin results.
Results
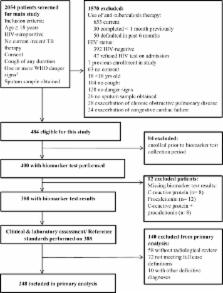
Two hundred forty-eight participants met study case definitions: 133 with tuberculosis, 61 with CAP, 16 with PJP, and 38 with mixed infection. In the 210 particpants with single infections the differences in median CRP and procalcitonin concentrations between the three infections were statistically significant, but distributions overlapped considerably. CRP and procalcitonin concentrations were highest in the CAP group and lowest in the PJP group. CRP and procalcitonin cut-offs with sensitivities of ≥90% were found for all three target infection pairs, but corresponding specificities were low. Highest receiver operating characteristic areas under the curve for CRP and procalcitonin were for PJP versus tuberculosis and PJP versus CAP (0.68 and 0.71, and 0.74 and 0.69 respectively).
Conclusions
CRP and procalcitonin showed limited value in discriminating between the three target infections due to widely overlapping distributions, but diagnostic accuracy was higher for discriminating PJP from CAP or tuberculosis. Our findings show limitations for CRP and procalcitonin, particularly for discriminiation of tuberculosis form CAP, however they may have greater diagnostic utility as part of a panel of biomarkers or in clinical prediction rules.
Related collections
Most cited references22

- Record: found
- Abstract: found
- Article: found
STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies
- Record: found
- Abstract: found
- Article: not found
Classification accuracy and cut point selection.
- Record: found
- Abstract: found
- Article: not found