- Record: found
- Abstract: found
- Article: found
Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial
Read this article at
Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the ongoing global pandemic known as COVID-19. Based on the potential antiviral role of quercetin, and on its described anti-blood clotting, anti-inflammatory and antioxidant properties, we hypothesize that subjects with mild COVID-19 treated with Quercetin Phytosome ® (QP), a novel bioavailable form of quercetin, may have a shorter time to virus clearance, a milder symptomatology, and higher probabilities of a benign earlier resolution of the disease.
Methods
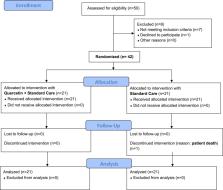
In our 2-week, randomized, open-label, and controlled clinical study, we have enrolled 42 COVID-19 outpatients. Twenty-one have been treated with the standard of care (SC), and 21 with QP as add-on supplementation to the SC. Our main aims were to check virus clearance and symptoms.
Results
The interim results reveal that after 1 week of treatment, 16 patients of the QP group were tested negative for SARS-CoV-2 and 12 patients had all their symptoms diminished; in the SC group, 2 patients were tested SARS-CoV-2 negative and 4 patients had their symptoms partially improved. By 2 weeks, the remaining 5 patients of the QP group tested negative for SARS-CoV-2, whereas in the SC group out of 19 remaining patients, 17 tested negatives by week 2, one tested negative by week 3 and one patient, still positive, expired by day 20. Concerning blood parameters, the add on therapy with QP, reduced LDH (−35.5%), Ferritin (−40%), CRP (−54.8%) and D-dimer (−11.9%).
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: found
The cytokine storm and COVID‐19
- Record: found
- Abstract: found
- Article: not found
Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy

- Record: found
- Abstract: found
- Article: found
