- Record: found
- Abstract: found
- Article: found
Slug, a Cancer‐Related Transcription Factor, is Involved in Vascular Smooth Muscle Cell Transdifferentiation Induced by Platelet‐Derived Growth Factor‐BB During Atherosclerosis
Read this article at
Abstract
Background
Heart attacks and stroke often result from occlusive thrombi following the rupture of vulnerable atherosclerotic plaques. Vascular smooth muscle cells ( VSMCs) play a pivotal role in plaque vulnerability because of their switch towards a proinflammatory/macrophage‐like phenotype when in the context of atherosclerosis. The prometastatic transcription factor Slug/Snail2 is a critical regulator of cell phenotypic transition. Here, we aimed to investigate the role of Slug in the transdifferentiation process of VSMCs occurring during atherogenesis.
Methods and Results
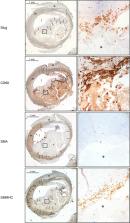
In rat and human primary aortic smooth muscle cells, Slug protein expression is strongly and rapidly increased by platelet‐derived growth factor‐BB (PDGF‐ BB). PDGF‐ BB increases Slug protein without affecting mRNA levels indicating that this growth factor stabilizes Slug protein. Immunocytochemistry and subcellular fractionation experiments reveal that PDGF‐ BB triggers a rapid accumulation of Slug in VSMC nuclei. Using pharmacological tools, we show that the PDGF‐ BB–dependent mechanism of Slug stabilization in VSMCs involves the extracellular signal‐regulated kinase 1/2 pathway. Immunohistochemistry experiments on type V and type VI atherosclerotic lesions of human carotids show smooth muscle–specific myosin heavy chain–/Slug‐positive cells surrounding the prothrombotic lipid core. In VSMCs, Slug si RNAs inhibit prostaglandin E2 secretion and prevent the inhibition of cholesterol efflux gene expression mediated by PDGF‐ BB, known to be involved in plaque vulnerability and/or thrombogenicity.
Conclusions
Our results highlight, for the first time, a role of Slug in aortic smooth muscle cell transdifferentiation and enable us to consider Slug as an actor playing a role in the atherosclerotic plaque progression towards a life‐threatening phenotype. This also argues for common features between acute cardiovascular events and cancer.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association.
- Record: found
- Abstract: found
- Article: not found
Smooth muscle cell fate and plasticity in atherosclerosis
- Record: found
- Abstract: found
- Article: not found