- Record: found
- Abstract: found
- Article: found
Risk factors for Hirschsprung disease-associated enterocolitis: a systematic review and meta-analysis
Read this article at
Abstract
Background:
The incidence of Hirschsprung disease (HSCR) is nearly 1/5000 and patients with HSCR are usually treated through surgical intervention. Hirschsprung disease-associated enterocolitis (HAEC) is a complication of HSCR with the highest morbidity and mortality in patients. The evidence on the risk factors for HAEC remains inconclusive to date.
Methods:
Four English databases and four Chinese databases were searched for relevant studies published until May 2022. The search retrieved 53 relevant studies. The retrieved studies were scored on the Newcastle–Ottawa Scale by three researchers. Revman 5.4 software was employed for data synthesis and analysis. Stata 16 software was employed for sensitivity analysis and bias analysis.
Results:
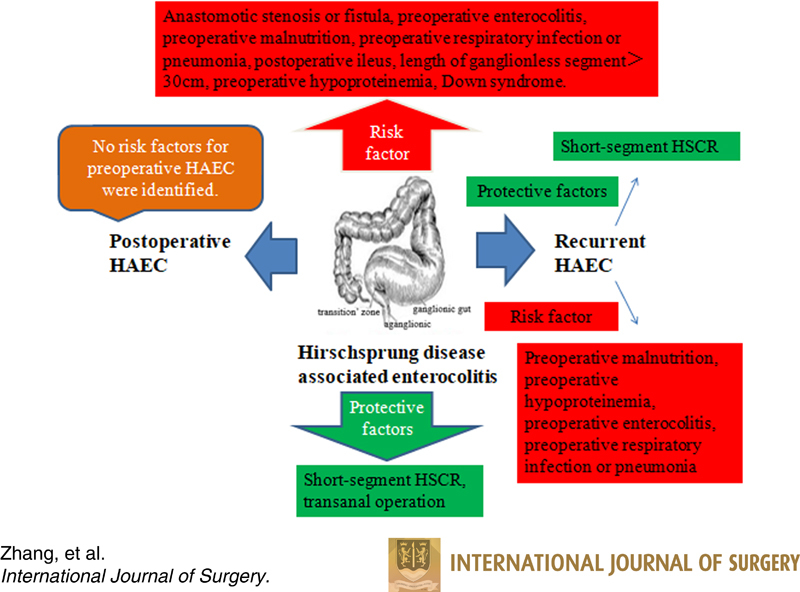
A total of 53 articles were retrieved from the database search, which included 10 012 cases of HSCR and 2310 cases of HAEC. The systematic analysis revealed anastomotic stenosis or fistula [ I 2=66%, risk ratio (RR)=1.90, 95% CI 1.34–2.68, P<0.001], preoperative enterocolitis ( I 2=55%, RR=2.07, 95% CI 1.71–2.51, P<0.001), preoperative malnutrition ( I 2=0%, RR=1.96, 95% CI 1.52–2.53, P<0.001), preoperative respiratory infection or pneumonia ( I 2=0%, RR=2.37, 95% CI 1.91–2.93, P<0.001), postoperative ileus ( I 2=17%, RR=2.41, 95% CI 2.02–2.87, P<0.001), length of ganglionless segment greater than 30 cm ( I 2=0%, RR=3.64, 95% CI 2.43–5.48, P<0.001), preoperative hypoproteinemia ( I 2=0%, RR=1.91, 95% CI 1.44–2.54, P<0.001), and Down syndrome ( I 2=29%, RR=1.65, 95% CI 1.32–2.07, P<0.001) as the risk factors for postoperative HAEC. Short-segment HSCR ( I 2=46%, RR=0.62, 95% CI 0.54–0.71, P<0.001) and transanal operation ( I 2=78%, RR=0.56, 95% CI 0.33–0.96, P=0.03) were revealed as the protective factors against postoperative HAEC. Preoperative malnutrition ( I 2=35 % , RR=5.33, 95% CI 2.68–10.60, P<0.001), preoperative hypoproteinemia ( I 2=20%, RR=4.17, 95% CI 1.91–9.12, P<0.001), preoperative enterocolitis ( I 2=45%, RR=3.51, 95% CI 2.54–4.84, P<0.001), and preoperative respiratory infection or pneumonia ( I 2=0%, RR=7.20, 95% CI 4.00–12.94, P<0.001) were revealed as the risk factors for recurrent HAEC, while short-segment HSCR ( I 2=0%, RR=0.40, 95% CI 0.21–0.76, P=0.005) was revealed as a protective factor against recurrent HAEC.
Abstract
Related collections
Most cited references90
- Record: found
- Abstract: not found
- Article: not found
Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses.
- Record: found
- Abstract: found
- Article: found
AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both
- Record: found
- Abstract: found
- Article: not found