- Record: found
- Abstract: found
- Article: found
Reduced Latency in the Metastatic Niche Contributes to the More Aggressive Phenotype of LM8 Compared to Dunn Osteosarcoma Cells
Read this article at
Abstract
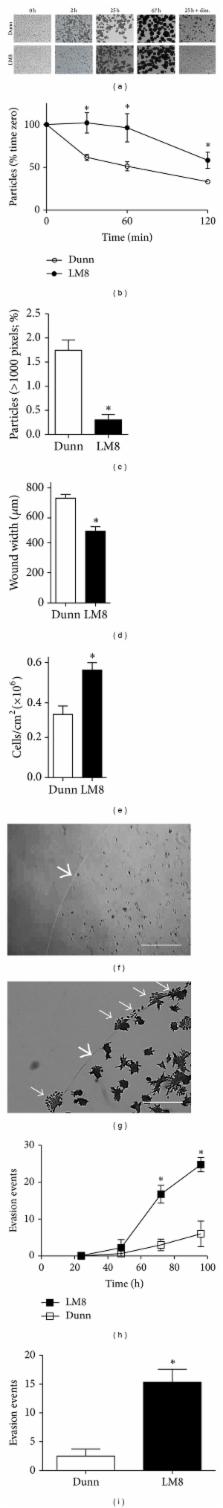
Metastasis is the major cause of death of osteosarcoma patients and its diagnosis remains difficult. In preclinical studies, however, forced expression of reporter genes in osteosarcoma cells has remarkably improved the detection of micrometastases and, consequently, the quality of the studies. We recently showed that Dunn cells equipped with a lacZ reporter gene disseminated from subcutaneous primary tumors as frequently as their highly metastatic subline LM8, but only LM8 cells grew to macrometastases. In the present time-course study, tail-vein-injected Dunn and LM8 cells settled within 24 h at the same frequency in the lung, liver, and kidney of mice. Furthermore, Dunn cells also grew to macrometastases, but, compared to LM8, with a delay of two weeks in lung and one week in liver and kidney tissue, consistent with prolonged survival of the mice. Dunn- and LM8-cell-derived ovary and spine metastases occurred less frequently. In vitro, Dunn cells showed less invasiveness and stronger contact inhibition and intercellular adhesion than LM8 cells and several cancer- and dormancy-related genes were differentially expressed. In conclusion, Dunn cells, compared to LM8, have a similar capability but a longer latency to form macrometastases and provide an interesting new experimental system to study tumor cell dormancy.
Related collections
Most cited references16
- Record: found
- Abstract: not found
- Article: not found
Molecular basis of metastasis.
- Record: found
- Abstract: found
- Article: not found
Genetic determinants of cancer metastasis.
- Record: found
- Abstract: found
- Article: not found