- Record: found
- Abstract: found
- Article: found
Id-1 Promotes Reendothelialization In The Early Phase After Vascular Injury Through Activation Of NFkB/survivin Signaling Pathway
Abstract
Background
Percutaneous coronary intervention (PCI) treatment can benefit patients, but also cause irreversible mechanical damage to the vascular endothelium, ultimately leading to restenosis of the target vessel. Thus, achieving rapid re-endothelialization and restoring the integrity of the vascular endothelium and function plays an important role in inhibiting neointimal hyperplasia and preventing restenosis. Id1 (inhibitor of DNA binding/differentiation factor 1) plays an important role in promoting cell proliferation and angiogenesis.
Study objective
This study aims to investigate the relationship between Id1 and NFκB/survivin signaling pathways and their role in injured vascular repair by establishing a rat carotid balloon injury model.
Methods
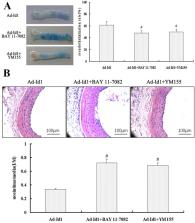
The carotid artery model of rat balloon injury was established. The injured common carotid artery was obtained at different time points after vascular injury. RNA and protein were extracted and the mRNA and protein expression levels of Id1, NFκB and survivin were detected in vascular injury. The NFκB blocker BAY 11–7082 and survivin blocker YM155 were used and the effects of Id1, NFκB, survivin mRNA and protein expression, revascularization of blood vessels and neointimal responsiveness after vascular injury were observed in the vascular tissues of Ad-Id1 transfected balloon injury.
Results
Id1, NFκB and survivin were expressed in injured rat carotid arteries. Overexpression of Id1 promoted re-endothelialization of injured vessels through NFκB/survivin signaling pathway, inhibited early vascular endometrial reactive hyperplasia; blocked NFκB the/survivin signaling pathway attenuates the re-endothelialization of Ad-Id1 and the early endothelium of Ad-Id1. Blocking the NFκB/survivin signaling pathway attenuates the re-endothelialization and early reactive hyperplasia of vascular intima of Ad-Id1.
Most cited references35
- Record: found
- Abstract: not found
- Article: not found
Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association
- Record: found
- Abstract: found
- Article: not found
Endothelial progenitor cells: characterization and role in vascular biology.
- Record: found
- Abstract: found
- Article: not found