- Record: found
- Abstract: found
- Article: found
The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/β-catenin signaling in human multiple myeloma
Read this article at
Abstract
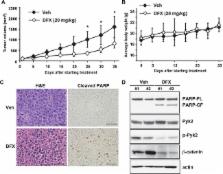
Deregulated iron metabolism underlies the pathogenesis of many human cancers. Recently, low expression of ferroportin, which is the only identified non-heme iron exporter, has been associated with significantly reduced overall survival in multiple myeloma (MM); however, the altered iron metabolism in MM biology remains unclear. In this study we demonstrated, by live cell imaging, that MM cells have increased intracellular iron levels as compared with normal cells. In experiments to test the effect of iron chelation on the growth of MM cells, we found that deferasirox (DFX), an oral iron chelator used to treat iron overload in clinical practice, inhibits MM cell growth both in vivo and in vitro. Mechanistically, DFX was found to induce apoptosis of MM cells via the inhibition of proline-rich tyrosine kinase 2 (Pyk2), which is known to promote tumor growth in MM. Inhibition of Pyk2 is caused by the suppression of reactive oxygen species, and leads to downregulation of the Wnt/β-catenin signaling pathway. Taken together, our findings indicate that high levels of intracellular iron, which might be due to low ferroportin expression, play a role in MM pathophysiology. Therefore, DFX may provide a therapeutic option for MM that is driven by deregulated iron homeostasis and/or Pyk2/Wnt signaling.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Iron and cancer: more ore to be mined.
- Record: found
- Abstract: found
- Article: not found
Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter.
- Record: found
- Abstract: found
- Article: not found