- Record: found
- Abstract: found
- Article: found
Fibroblast Growth Factor Receptor (FGFR) Inhibitors in Urothelial Cancer
Read this article at
Abstract
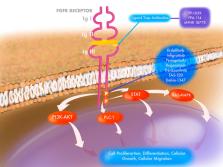
Dysregulated fibroblast growth factor receptor (FGFR) signaling is associated with several cancers, including urothelial carcinoma. Preclinical studies with FGFR inhibitors have shown significant antitumor activity, which has led to clinical evaluation of multiple FGFR inhibitors. Recently, erdafitinib was approved by the U.S. Food and Drug Administration for advanced urothelial carcinoma with FGFR gene alterations as the first molecularly targeted therapy. Additional ongoing clinical trials with other types of FGFR inhibitors have shown encouraging results. This review summarizes the oncogenic signaling of FGFR alterations, completed and ongoing clinical trials of FGFR inhibitors, and resistance patterns.
Implications for Practice
Dysregulated fibroblast growth factor receptor (FGFR) signaling is associated with several cancers, including urothelial carcinoma. Preclinical studies with FGFR inhibitors have shown significant antitumor activity, which has led to clinical evaluation of multiple FGFR inhibitors. Most recently, erdafitinib was approved by the U.S. Food and Drug Administration for advanced urothelial carcinoma with FGFR gene alterations as the first molecularly targeted therapy. Additional ongoing clinical trials with other types of FGFR inhibitors have shown encouraging results. This review summarizes the oncogenic signaling of FGFR alterations, completed and ongoing clinical trials of FGFR inhibitors, and resistance patterns.
Abstract
Focusing on urothelial malignancies, this review summarizes the oncogenic signaling of FGFR alterations, clinical trials of FGFR inhibitors, and resistance patterns.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2020
- Record: found
- Abstract: found
- Article: not found
AACR Project GENIE: Powering Precision Medicine through an International Consortium
- Record: found
- Abstract: found
- Article: not found