- Record: found
- Abstract: found
- Article: found
FDG-PET/CT in indeterminate thyroid nodules: cost-utility analysis alongside a randomised controlled trial
Read this article at
Abstract
Purpose
To evaluate cost-effectiveness of an [ 18F]FDG-PET/CT-driven diagnostic workup as compared to diagnostic surgery, for thyroid nodules with Bethesda III/IV cytology. [ 18F]FDG-PET/CT avoids 40% of futile diagnostic surgeries for benign Bethesda III/IV nodules.
Methods
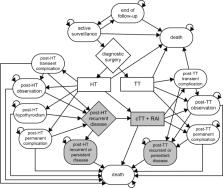
Lifelong societal costs and quality-adjusted life years (QALYs) were assessed for 132 patients participating in a randomised controlled multicentre trial comparing [ 18F]FDG-PET/CT to diagnostic surgery. The observed 1-year trial results were extrapolated using a Markov model. The probability of cost-effectiveness was estimated using cost-effectiveness acceptability curves, taking uncertainty about sampling, imputation, and parameters into account.
Results
The observed 1-year cost difference of [ 18F]FDG-PET/CT as compared to diagnostic surgery was − €1000 (95% CI: − €2100 to €0) for thyroid nodule–related care (p = 0.06). From the broader societal perspective, the 1-year difference in total societal costs was − €4500 (− €9200 to €150) (p = 0.06). Over the modelled lifelong period, the cost difference was − €9900 (− €23,100 to €3200) (p = 0.14). The difference in QALYs was 0.019 (− 0.045 to 0.083) at 1 year (p = 0.57) and 0.402 (− 0.581 to 1.385) over the lifelong period (p = 0.42). For a willingness to pay of €50,000 per QALY, an [ 18F]FDG-PET/CT-driven work-up was the cost-effective strategy with 84% certainty.
Conclusion
Following the observed reduction in diagnostic surgery, an [ 18F]FDG-PET/CT-driven diagnostic workup reduced the 1-year thyroid nodule–related and societal costs while sustaining quality of life. It is very likely cost-effective as compared to diagnostic surgery for Bethesda III/IV nodules.
Trial registration number: This trial is registered with ClinicalTrials.gov: NCT02208544 (5 August 2014), https://clinicaltrials.gov/ct2/show/NCT02208544.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer.
- Record: found
- Abstract: found
- Article: not found
Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)
- Record: found
- Abstract: found
- Article: not found