- Record: found
- Abstract: found
- Article: found
Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives
Read this article at
Abstract
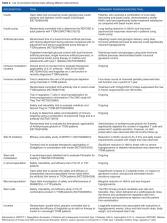
Type 1 diabetes (T1D) is caused by autoimmune destruction of insulin-producing β cells located in the endocrine pancreas in areas known as islets of Langerhans. The current standard-of-care for T1D is exogenous insulin replacement therapy. Recent developments in this field include the hybrid closed-loop system for regulated insulin delivery and long-acting insulins. Clinical studies on prediction and prevention of diabetes-associated complications have demonstrated the importance of early treatment and glucose control for reducing the risk of developing diabetic complications. Transplantation of primary islets offers an effective approach for treating patients with T1D. However, this strategy is hampered by challenges such as the limited availability of islets, extensive death of islet cells, and poor vascular engraftment of islets post-transplantation. Accordingly, there are considerable efforts currently underway for enhancing islet transplantation efficiency by harnessing the beneficial actions of stem cells. This review will provide an overview of currently available therapeutic options for T1D, and discuss the growing evidence that supports the use of stem cell approaches to enhance therapeutic outcomes.
Related collections
Most cited references112
- Record: found
- Abstract: found
- Article: not found
Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo.
- Record: found
- Abstract: not found
- Article: not found
Type I diabetes mellitus. A chronic autoimmune disease.

- Record: found
- Abstract: found
- Article: found
