- Record: found
- Abstract: found
- Article: found
Robotic coronary revascularization in Europe, state of art and future of EACTS-endorsed Robotic Cardiothoracic Surgery Taskforce
editorial

Matto Pettinari
1
,
,
Monica Gianoli
2 ,
Meindert Palmen
3 ,
Stepan Cerny
4 ,
Burak Onan
5 ,
Sandeep Singh
6 ,
Patrique Segers
7 ,
Cengiz Bolcal
8 ,
Cem Alhan
9 ,
Emiliano Navarra
10 ,
Herbert De Praetere
11 ,
Jan Vojacek
12 ,
Theodor Cebotaru
13 ,
Paul Modi
14 ,
Fabien Doguet
15 ,
Ulrich Franke
16 ,
Ahmed Ouda
17 ,
Ludovic Melly
18 ,
Ghislain Malapert
19 ,
Louis Labrousse
20 ,
Alfonso Agnino
21 ,
Tine Philipsen
22 ,
Jean-Luc Jansens
23 ,
Thierry Folliguet
24 ,
Daniel Pereda
25 ,
Francesco Musumeci
26 ,
Piotr Suwalski
27 ,
Koen Cathenis
28 ,
Frank Van Praet
29 ,
Johannes Bonatti
30 ,
Wouter Oosterlinck
31
the European Robotic CardioThoracic Surgeons (ERCTS)
18 May 2022
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
BACKGROUND
It has been >20 years ago that robotic-assisted coronary artery bypass grafting (RA-CABG)
has been introduced, but the adoption of this technique is still rather limited worldwide,
although recently a slight increase in numbers has been documented in Europe [1, 2].
Like many novelties, after the introduction, it has been picked up by only a few dedicated
surgeons in highly specialized centres. Due to limited series, based mostly on single-centre
experiences, extensive clinical outcome data and results on long-term benefits are
lacking as well as the acknowledgement in international cardiosurgical society and
anchorage in EACTS-supported guidelines. The limited number of robotic platforms and
high procedural costs combined with the absence of dedicated training programs are
considered to be responsible for reduced adoption. The safety of robotic techniques,
the benefit of the left internal mammary artery (LIMA) to left anterior descending
(LAD) over percutaneous coronary interventions (PCI) and hybrid procedures have also
been questioned. Nonetheless, after 20 years, the robotic surgical technique has evolved.
Consequentially, the number of off-pump robot-assisted minimally invasive direct coronary
artery bypass (RA-MIDCAB) has rapidly grown lately [1, 2]. It seems that the robotic
approach to ischaemic heart disease has earned its place in our surgical armamentarium.
This editorial will address the current standards of care and future perspectives
of robotics in coronary revascularization.
ROBOTIC-ASSISTED CORONARY REVASCULARIZATION APPROACHES
The first robotic-assisted coronary revascularization was described in 1999 by Loulmet.
In 6 patients, the left internal mammary artery (IMA) was harvested using a robotic
approach and subsequently grafted to the LAD coronary artery. In 2 patients, the procedure
was performed completely endoscopically. Recently, we witnessed a growing interest
in minimally invasive coronary artery bypass grafting (CABG), performed not only robotically
assisted but also under ‘direct view’ or videoscopy assisted. Nevertheless, non-robotic
procedures showed mostly inferior outcomes when compared to the robotic ones, in terms
of major acute cardiac and cerebrovascular events (MACCE), duration of intensive care
unit stay and postoperative pain. In a recent study, Bonatti et al. [3] reviewed the
25-year-long journey of minimally invasive coronary surgery, demonstrating how robotic
activity increased after the FDA approval of the Da Vinci system (Intuitive Surgical,
Sunnyvale, CA, USA) in 2000. Afterward, fluctuation in the number of performed procedures
showed a first peak in 2006 and the second one in 2014 (Fig. 1). In Europe, where
robotic surgery did not reach the popularity achieved in the USA, probably due to
the differences in the economical asset of the Public Health System, we recently witnessed
a doubling in the numbers of centres performing robotic coronary revascularization
between 2016 and 2019. Maintaining the same rate of growth, we expect that the European
robotic CABG volume could equal the US volume in the next 5 years [2].
Figure 1
Number of patients treated by minimally invasive coronary artery bypass derived from
the published literature available.
Essentially, 2 different coronary revascularization procedures can be performed using
the robotic platform: RA-MIDCAB and totally endoscopic coronary artery bypass graft
(TECAB). In RA-MIDCAB, surgeons use the robotic system to harvest 1 or 2 IMA’s, open
the pericardium and identify coronary targets. Through a small left anterior thoracotomy,
coronary anastomosis is manually crafted in an off-pump setting. The second procedure,
TECAB, is completely robotically performed, and therefore technically challenging
for the surgeons. Without additional thoracotomy, target vessel stabilization and
grafting are completely performed endoscopically. TECAB in its beating heart version
is only feasible using a robotic endostabilizer. Also, robotic suturing of the anastomosis
is challenging and automatic connector devices have been developed. Unfortunately,
both technologies due to the lack of demand have been on hold which prevents the further
spread of this procedure. A few centres still perform it using work around but RA-MIDCAB
is currently the most adopted technique.
COMPARISON TO CONVENTIONAL CABG
Since Loulmet’s first report, clinical outcomes after robotic CABG were obtained mostly
from single-centre retrospective observational data. Several series showed excellent
results with a low incidence of mortality, stroke and myocardial infarction. Robotic
techniques also showed a reduction in pneumonia, postoperative pain, transfusion requirement
and recovery time when compared to conventional CABG. Bonatti’s review [3] on 11 135
patients reported hospital mortality of 1% and a stroke rate of 0.6%. The revision
rate for bleeding was 2.5% and a renal failure rate of 0.9% was noted. Wound infections
occurred at a rate of 1.2% and postoperative hospital stay was close to 5 days. An
average of 1.3 grafts were performed in <4 h of operative time adopting 6 main versions
of minimal access and robotically assisted CABG. The review concluded that less invasive
and robotically assisted versions of coronary bypass grafting are carried out with
an adequate safety level while surgical trauma is significantly reduced when compared
to standard CABG. Also, current European outcomes for robotic CABG, on 1266 patients,
are comparatively very encouraging with very low mortality (0.6%) and no strokes (Fig. 2).
Revision for bleeding rate of 2.1% is acceptable and the low (2.6%) conversion rate
likely reflects a learning curve of the robotic cardiac surgery community and demonstrates
that the procedures have become more standardized [2]. A further recent meta-analysis
comparing TECAB and RA-MIDCAB to conventional CABG, demonstrated a reduction at 1
year of the composite outcome of death, myocardial infarction and stroke in favour
of the robotic procedures. Also, outcomes such as graft patency and the need for repeat
revascularization (RR) were excellent. In literature, a similar rate of RR for the
2 procedures is reported, demonstrating that robotic CABG meets the standards of open
CABG concerning graft quality. Most of the RA-MIDCAB or TECAB procedures were performed
for single-vessel disease; however, experienced teams demonstrated the feasibility
of performing multiple arterial bypass using both IMAs, with an average of 2.4 anastomosis/patient,
in multivessel disease [4]. In Balkhy series, the right internal mammary artery (RIMA)
was used as an in situ graft in 124 cases (84%) and as a free T-graft in 24 cases
(16%) cases. The use of bilateral mammary artery increased from 23% in the first 5 years
to 53% in the last 2 years. Also, for these complex procedures, perioperative mortality
and morbidity were low. Mortality was 0.7%, myocardial infarction 0.3–1.1% and stroke
0.5%. Length of hospital stay was quite short reporting an average of 3 days. The
authors concluded that robotic TECAB allows the routine harvesting and use of the
RIMA graft in a safe and reproducible manner. In the last years, besides the implementation
of surgical strategy with the adoption of completely arterial revascularization for
the left coronaries, the complexity of the patient referred to robotic revascularization
increased. Obesity, elderly, redo operation or chronic pulmonary diseases in the past
considered as a contra-indication for MIDCAB and TECAB became lately more common characteristics
among the robotic population [4]. In fact, despite an intrinsic increased operative
risk, those patients are the most advantaged by a sternal sparing approach and an
early recovery.
Figure 2
Expected versuss observed mortality after coronary and robotic procedure. Taken from
Cerny et al. [2].
THE BENEFIT OF THE LEFT INTERNAL MAMMARY ARTERY–LEFT ANTERIOR DESCENDING OVER PERCUTANEOUS
CORONARY INTERVENTIONS
CABG and PCI are well-established revascularization strategies for proximal LAD lesions,
both are considered as the first option in the European guidelines for revascularization.
However, even after 2 decades, minimally invasive surgical revascularization has never
been included in the general recommendations. Surgical revascularization (LITA to
LAD) offers a better long-term survival and decreased demand for RR, while PCI offers
a less-invasive nature of the treatment. PCI represents a valuable alternative for
old and multimorbid patients with high risk for surgery or simply a temporary solution
to delay surgery in young and still fit patients. In the past, the SIMA trial showed
the superiority of the mammary artery when compared to the bare-metal stent in terms
of RR up to 10 years. Lately, the introduction of drug-eluting stents (DES) has changed
the equation somewhat during the last decade. Although DES reduced the incidence of
early restenosis, its inferiority compared to CABG was demonstrated in several meta-analyses
[5]. Outcomes in these studies were congruent: mortality and MACCE were similar in
both groups, while the need for RR was higher using DES. The second generation of
DES reduced the need for RR, but even when PCI was performed FFR guided, targeting
only the functionally significant lesions and avoiding unnecessary stenting and herewith
stent-related complications, the occurrence of MACCE within 1 year was higher in the
PCI group when compared to CABG [6]. For isolated LAD lesions, minimally invasive
surgical revascularization with IMA to LAD showed lower RR, and higher freedom from
angina especially when a longer stent (>30 mm) was deemed necessary with percutaneous
revascularization. Similar findings were described for left main disease, by a recent
meta-analysis, demonstrating lower rates of late target vessel RR in patients undergoing
MIDCAB when compared to PCI [7]. In experienced robotic teams, bilateral IMA harvesting
and robot-assisted target vessel revascularization of the left-sided coronary lesions
could further improve outcomes and revascularization options. In addition, skeletonization
and sternal sparing allow the RIMA to reach various coronary targets [4]. In this
setting, the patients receive the advantages of completely arterial revascularization
with the benefit of a less-invasive approach. Robotically assisted placement of bilateral
IMAs and combination with PCI in advanced hybrid coronary revascularization for the
complex multivessel disease has also been successfully carried out. In fact, the use
of mammary arteries for surgical revascularization may have specific advantages when
compared to PCI, which can be attributed to their specific anatomical and biological
characteristics. IMAs produce a high level of nitric oxide inducing endothelial-dependent
vasodilation effect also in the grafted coronaries and providing a ‘surgical collateralization’,
prolonging life by preventing myocardial infarction [8]. Although most of these considerations
indicate the need for surgical revascularization of, at least the more complex, LAD
lesions, inappropriate or traumatic IMA graft harvesting techniques could easily impair
graft patency and therefore outcome [9]. Nowadays, robotic-assisted harvesting of
the ITAs can be performed with minimal tissue damage (Fig. 3), resulting in optimal
graft patency while reducing complications like (sternal) wound infections [3, 4].
Furthermore, a more extensive intraoperative graft quality control using a Transit
Time Flowmeter, highly recommended during minimally invasive CABG, permits direct
analysis of the final results with the aim to improve early and late graft patency.
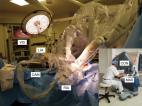
Figure 3
Intraoperative view of left mammary artery harvesting.
HYBRID REVASCULARIZATION
Hybrid coronary revascularization (HCR) combines surgical coronary revascularization
(LIMA-to-LAD graft) with percutaneous coronary revascularization (PCI of significantly
affected non-LAD lesions). Robotic-assisted techniques enabling LITA-to-LAD grafting
provide the patient with the survival benefit of the LITA–LAD grafting while avoiding
the risks of cardiopulmonary bypass, aortic manipulations and sternotomy. Furthermore,
integrated PCIs provide the patient with the least invasive HCR option, achieving
complete revascularization of all diseased coronary arteries. The use of the second-generation
DES is of paramount importance in the treatment of non-LAD coronary pathology and
provides a valuable alternative to surgical revascularization of non-LAD targets using
a venous graft, the latter being notorious for future atherosclerotic degeneration
resulting in high short- and long-term failure rates [5–7].
Several single-centre studies comparing HCR to CABG have been published so far. Improvements
in short-term outcomes in terms of hospital stay and transfusion requirements have
been described in favour of HCR. Long-term data demonstrated at nearly 10-year follow-up
similar outcomes in terms of composite end-point of death, RR and new myocardial infarction.
Clear data comparing HCR and total arterial open CABG are still lacking in the literature
and there is definitely a need for prospective randomized comparisons.
In conclusion, the ideal candidates for (robotically-assisted) HCR could be patients
with multivessel disease with a complex LAD lesion suitable for LIMA–LAD grafting,
associated with non-complex non-LAD lesions (SYNTAX score <22) suitable for PCI. Importantly,
HCR should not be considered as an alternative to CABG for patients with diffuse complex
coronary pathology (SYNTAX >22) but should be viewed as an alternative to multivessel
PCI in patients with LAD disease having low-intermediate SYNTAX score. The more complex
disease may be amenable to advanced hybrid revascularization concepts including robotic
double IMA grafting for the left coronaries and PCI for the right side. Nevertheless,
each patients’ specific decision needs to be discussed by the heart team to define
the most appropriate tailored approach.
TRAINING AND QUALITY CONTROL
RA-CABG represents roughly 1–3% of total CABG procedures performed in Europe [1].
Reasons for limited adoption might include high initial investment and high procedural
costs of the robotic platform and the demand for a high level of expertise for all
teams involved in the procedure. The lack of a formalized training program also plays
an important role. In 2016, a joint Society of Thoracic Surgeons and American Association
for Thoracic Surgery task force was created to address the gaps in RA-CABG adoption
and performance implementation. Optimal surgeon training has been identified as a
critical component of procedural development across various domains. The single-centre
series evaluated the effect of the level of surgical experience on the efficiency
of the procedures. It was shown that between 5 and 20 cases, IMA harvesting time decreased
significantly. Similar trends were observed for the time needed for port placement
and coronary artery grafting and consequently for the overall operative procedural
duration [10]. Surgeons’ learning curve may potentially also affect procedural success.
Although the steepness of the learning curve may vary amongst surgeons, it has been
described that in experienced teams with more than 50 procedures, a decreased (decrease)
in conversion rate, reoperation need and mortality can be observed [10]. Beating heart
off-pump surgical revascularization skills and a dedicated team approach, may also
shorten this learning curve, allowing for safe implementation and paving the road
towards more complex procedures such as multivessel completely arterial revascularization.
Benchmarking RA-CABG outcomes, creating both a nationwide and an international registry,
is considered to be a necessary step to guarantee quality control. Apart from benchmarking
and quality control, a registry may allow for a large retrospective cohort study comparing
RA-CABG with both conventional CABG and multivessel PCIs. Furthermore, we expect that
a standard of reference will also improve the performances of the individual robotic
centres.
CONCLUSION
Robotic CABG has been adopted slowly after its initial introduction more than 2 decades
ago but gained popularity in the past few years. Being an ideal surgical counterpart
for PCI in HCR strategies, we expect that robotic CABG may contribute to a paradigm
shift in the treatment of patients with complex multivessel coronary artery disease.
Visibility and acceptance of robotic CABG in myocardial revascularization guidelines,
the set-up of official international training programmes, procedural benchmarking
and active involvement of the international cardiothoracic society are crucial but
still lacking to date. The first step towards acknowledgement of the role of robotics
in cardiac surgery was taken by the European Society of Thoracic and Cardiovascular
Surgery, which supported the implementation of an EACTS-endorsed Robotic Cardiothoracic
Surgery Taskforce. The aim of this task force is to analyse actual and future outcomes,
promote high-quality team training, stimulate support from the industry and improve
the application of future technologies.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
PCI and CABG for Treating Stable Coronary Artery Disease
- Record: found
- Abstract: found
- Article: not found
Fractional Flow Reserve–Guided PCI as Compared with Coronary Bypass Surgery
- Record: found
- Abstract: found
- Article: found