- Record: found
- Abstract: found
- Article: found
Trpm4 Gene Invalidation Leads to Cardiac Hypertrophy and Electrophysiological Alterations
Read this article at
Abstract
Rationale
TRPM4 is a non-selective Ca 2+-activated cation channel expressed in the heart, particularly in the atria or conduction tissue. Mutations in the Trpm4 gene were recently associated with several human conduction disorders such as Brugada syndrome. TRPM4 channel has also been implicated at the ventricular level, in inotropism or in arrhythmia genesis due to stresses such as ß-adrenergic stimulation, ischemia-reperfusion, and hypoxia re-oxygenation. However, the physiological role of the TRPM4 channel in the healthy heart remains unclear.
Objectives
We aimed to investigate the role of the TRPM4 channel on whole cardiac function with a Trpm4 gene knock-out mouse ( Trpm4 -/-) model.
Methods and Results
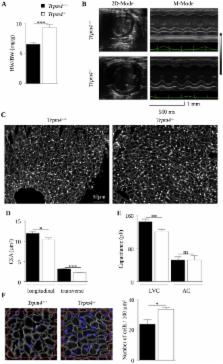
Morpho-functional analysis revealed left ventricular (LV) eccentric hypertrophy in Trpm4 -/- mice, with an increase in both wall thickness and chamber size in the adult mouse (aged 32 weeks) when compared to Trpm4 +/+ littermate controls. Immunofluorescence on frozen heart cryosections and qPCR analysis showed no fibrosis or cellular hypertrophy. Instead, cardiomyocytes in Trpm4 -/- mice were smaller than Trpm4 +/+ with a higher density. Immunofluorescent labeling for phospho-histone H3, a mitosis marker, showed that the number of mitotic myocytes was increased 3-fold in the Trpm4 -/- neonatal stage, suggesting hyperplasia. Adult Trpm4 -/- mice presented multilevel conduction blocks, as attested by PR and QRS lengthening in surface ECGs and confirmed by intracardiac exploration. Trpm4 -/- mice also exhibited Luciani-Wenckebach atrioventricular blocks, which were reduced following atropine infusion, suggesting paroxysmal parasympathetic overdrive. In addition, Trpm4 -/- mice exhibited shorter action potentials in atrial cells. This shortening was unrelated to modifications of the voltage-gated Ca 2+ or K + currents involved in the repolarizing phase.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development.
- Record: found
- Abstract: found
- Article: not found
TRP-PLIK, a bifunctional protein with kinase and ion channel activities.
- Record: found
- Abstract: found
- Article: not found