- Record: found
- Abstract: found
- Article: found
Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy
Read this article at
Abstract
Objective
To investigate the role of inflammation in the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy (HCM).
Subjects
Twenty-four patients with a single HCM-causing mutation D175N in the α-tropomyosin gene and 17 control subjects.
Main outcome measures
Endomyocardial biopsy samples taken from the patients with HCM were compared with matched myocardial autopsy specimens. Levels of high-sensitivity C-reactive protein (hsCRP) and proinflammatory cytokines were measured in patients and controls. Myocardial late gadolinium enhancement (LGE) in cardiac MRI (CMRI) was detected.
Results
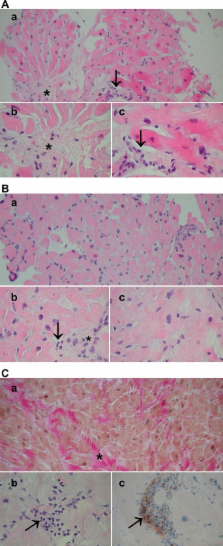
Endomyocardial samples in patients with HCM showed variable myocyte hypertrophy and size heterogeneity, myofibre disarray, fibrosis, inflammatory cell infiltration and nuclear factor kappa B (NF-κB) activation. Levels of hsCRP and interleukins (IL-1β, IL-1RA, IL-6, IL-10) were significantly higher in patients with HCM than in control subjects. In patients with HCM, there was a significant association between the degree of myocardial inflammatory cell infiltration, fibrosis in histopathological samples and myocardial LGE in CMRI. Levels of hsCRP were significantly associated with histopathological myocardial fibrosis. hsCRP, tumour necrosis factor α and IL-1RA levels had significant correlations with LGE in CMRI.
Conclusions
A variable myocardial and systemic inflammatory response was demonstrated in patients with HCM attributable to an identified sarcometric mutation. Inflammatory response was associated with myocardial fibrosis, suggesting that myocardial fibrosis in HCM is an active process modified by an inflammatory response.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Hypertrophic cardiomyopathy: a systematic review.
- Record: found
- Abstract: found
- Article: not found
Inflammatory Mediators and the Failing Heart: Past, Present, and the Foreseeable Future
- Record: found
- Abstract: found
- Article: not found