- Record: found
- Abstract: found
- Article: found
Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments
Read this article at
Abstract
Background
Nanoemulsions have practical application in a multitude of commercial areas, such as the chemical, pharmaceutical and cosmetic industries. Cosmetic industries use rice bran oil in sunscreen formulations, anti ageing products and in treatments for skin diseases. The aim of this study was to create rice bran oil nanoemulsions using low energy emulsification methods and to evaluate their physical stability, irritation potential and moisturising activity on volunteers with normal and diseased skin types.
Results
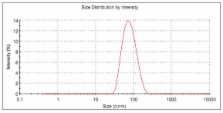
The nanoemulsion developed by this phase diagram method was composed of 10% rice bran oil, 10% surfactants sorbitan oleate/PEG-30 castor oil, 0.05% antioxidant and 0.50% preservatives formulated in distilled water. The nanoemulsion was stable over the time course of this study. In vitro assays showed that this formulation has a low irritation potential, and when applied to human skin during in vivo studies, the nanoemulsion improved the skin's moisture and maintained normal skin pH values.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
New insights into atopic dermatitis.
- Record: found
- Abstract: not found
- Article: not found
Re-coalescence of emulsion droplets during high-energy emulsification
- Record: found
- Abstract: found
- Article: not found