- Record: found
- Abstract: found
- Article: found
C3-glomerulonephritis in New Zealand – a case series
Read this article at
Abstract
Background
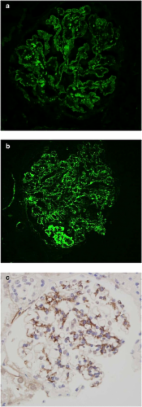
C3-glomerulonephritis can lead to progressive renal impairment from complement-mediated glomerular injury. Incidence and outcomes of C3-glomerulonephritis are not known in the New Zealand population.
Methods
We reviewed all cases of C3-glomerulonephritis from the past 10 years at a tertiary referral centre in New Zealand. Descriptive information on baseline characteristics and clinical outcomes was collected.
Results
Twenty-six patients were included (16 men; mean ± SD age 44 ± 25 years) with a median follow-up of 30 months. Disease incidence was 1.3 cases per million individuals, of which 42% were Pacific Islanders. Most patients presented with renal impairment, with a median (IQR) creatinine at diagnosis of 210 (146–300) μmol/L, and 11 (42%) patients presented with nephrotic syndrome. Seven (27%) patients progressed to end stage renal disease and 2 (8%) had died. End stage renal disease occurred in 20% of patients treated with immunosuppression and in 50% of those not treated. Complete remission was seen in 25% of patients treated with some form of immunosuppression and in 17% of those not treated.
Conclusions
Our results are consistent with previous descriptions of C3-glomerulonephritis. There was a suggestion of better clinical outcomes in patients treated with immunosuppression. There was a higher disease incidence in Pacific Islanders, which may indicate an underlying susceptibility to complement dysfunction in this population.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Eculizumab for dense deposit disease and C3 glomerulonephritis.
- Record: found
- Abstract: found
- Article: not found