- Record: found
- Abstract: found
- Article: found
Intravitreal brolucizumab as treatment of early onset radiation retinopathy secondary to plaque brachytherapy for choroidal melanoma
Read this article at
Abstract
Purpose
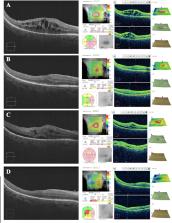
To describe the efficacy and safety of brolucizumab (Beovu®, Novartis Pharmaceuticals) in a case of cystoid macular edema associated with radiation retinopathy as a result of iodine-125 plaque brachytherapy (PBT) for choroidal melanoma, resistant to treatment with other anti-vascular endothelial growth factor (VEGF) agents.
Observations
A 67-year-old woman with choroidal melanoma in the right eye and best-corrected visual acuity (BCVA) of 20/20, underwent uncomplicated PBT. On post-operative month 7, the patient developed early onset radiation retinopathy. She failed to improve significantly with sub-tenon triamcinolone and 3 injections of intravitreal bevacizumab; BCVA was 20/200. Intravitreal brolucizumab was administered, and one month after, macular edema had resolved completely on optical coherence tomography, and BCVA improved to 20/50. At last follow up, 1 month after the third brolucizumab injection, BCVA was 20/60 and there was sustained resolution of intraretinal fluid. There were no signs of intraocular inflammation, progressive RR or optic neuropathy on exam or fluorescein angiography.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration
- Record: found
- Abstract: found
- Article: not found
Occlusive Retinal Vasculitis Following Intravitreal Brolucizumab

- Record: found
- Abstract: found
- Article: found