- Record: found
- Abstract: found
- Article: found
Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells
Read this article at
Abstract
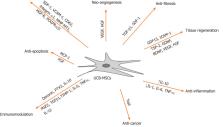
Umbilical cord blood (UCB) is a primitive and abundant source of mesenchymal stem cells (MSCs). UCB-derived MSCs have a broad and efficient therapeutic capacity to treat various diseases and disorders. Despite the high latent self-renewal and differentiation capacity of these cells, the safety, efficacy, and yield of MSCs expanded for ex vivo clinical applications remains a concern. However, immunomodulatory effects have emerged in various disease models, exhibiting specific mechanisms of action, such as cell migration and homing, angiogenesis, anti-apoptosis, proliferation, anti-cancer, anti-fibrosis, anti-inflammation and tissue regeneration. Herein, we review the current literature pertaining to the UCB-derived MSC application as potential treatment strategies, and discuss the concerns regarding the safety and mass production issues in future applications.
Related collections
Most cited references149
- Record: found
- Abstract: found
- Article: not found
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement.
- Record: found
- Abstract: found
- Article: not found
Concise review: the surface markers and identity of human mesenchymal stem cells.
- Record: found
- Abstract: found
- Article: not found