- Record: found
- Abstract: found
- Article: found
The fatty acid binding protein 6 gene ( Fabp6) is expressed in murine granulosa cells and is involved in ovulatory response to superstimulation
Read this article at
Abstract
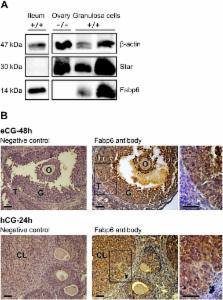
The fatty acid binding protein 6 (Fabp6) is commonly regarded as a bile acid binding protein found in the distal portion of the small intestine and has been shown to be important in maintaining bile acid homeostasis. Previous studies have also reported the presence of Fabp6 in human, rat and fish ovaries, but the significance of Fabp6 in this organ is largely unknown. Therefore, we surveyed murine ovaries for Fabp6 gene expression and evaluated its role in ovarian function using mice with whole body Fabp6 deficiency. Here we show that the Fabp6 gene is expressed in granulosa and luteal cells of the mouse ovary. Treatment with gonadotropins stimulated Fabp6 gene expression in large antral follicles. The ovulation rate in response to superovulatory treatment in Fabp6-deficient mice was markedly decreased compared to wildtype (C57BL/6) mice. The results of this study suggest that expression of Fabp6 gene in granulosa cells serves an important and previously unrecognized function in fertility.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases.
- Record: found
- Abstract: found
- Article: not found
Liver receptor homolog 1 is essential for ovulation.
- Record: found
- Abstract: found
- Article: not found