- Record: found
- Abstract: found
- Article: found
Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: A bioavailable turmeric formulation
Read this article at
Abstract
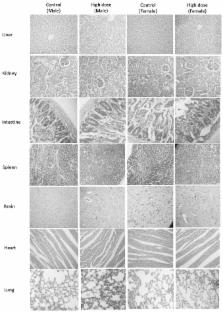
Curcumin, the active component present in Curcuma longa of the family Zingiberaceae, has a number of pharmacological effects, including potential anti-inflammatory activity. One of the major limitations of curcumin/turmeric extract is its poor absorption through the gastrointestinal tract. Several approaches have been adopted to increase the bioavailability of curcumin, including loading curcumin into liposomes or nanoparticles, complexation with phospholipids, addition of essential oils and synthesizing structural analogues of curcumin. In the present study, the toxicity and safety of one such bioavailable turmeric formulation, curcuminoid-essential oil complex (CEC), the toxicity profile of which has not been reported, were examined using in vivo and in vitro models, as per the guidelines of the Organisation for Economic Co-operation and Development. Investigations of acute toxicity study were performed in rats and mice, and the results revealed no signs and symptoms or toxicity or mortality in any of the animals at the maximum recommended dose level of 5,000 mg/kg body weight. The repeated administration of CEC for 90 days in Wistar rats at a dose of 1,000 mg/kg body weight did not induce any observable toxic effects, compared with corresponding control animals. Mutagenicity/genotoxicity investigations were also performed using a bacterial reverse mutation assay (Ames test), a mammalian bone marrow chromosome aberration test and a mammalian erythrocyte micronucleus test in mice. CEC was found to be non-mutagenic in all three mutagenic investigations. Consequently, the present study indicated that CEC elicited no toxic effects in animals or in vitro. Therefore, following investigations of acute toxicity, repeated dose toxicity and mutagenicity, CEC was deemed a safe, non-toxic pharmacological formulation.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Multiple biological activities of curcumin: a short review.
- Record: found
- Abstract: found
- Article: not found