- Record: found
- Abstract: found
- Article: not found
Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET
Abstract
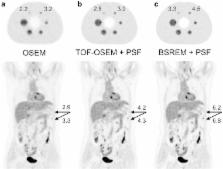
In recent years, there have been multiple advances in positron emission tomography/computed tomography (PET/CT) that improve cancer imaging. The present generation of PET/CT scanners introduces new hardware, software, and acquisition methods. This review describes these new developments, which include time-of-flight (TOF), point-spread-function (PSF), maximum-a-posteriori (MAP) based reconstruction, smaller voxels, respiratory gating, metal artefact reduction, and administration of quadratic weight-dependent 18F–fluorodeoxyglucose (FDG) activity. Also, hardware developments such as continuous bed motion (CBM), (digital) solid-state photodetectors and combined PET and magnetic resonance (MR) systems are explained. These novel techniques have a significant impact on cancer imaging, as they result in better image quality, improved small lesion detectability, and more accurate quantification of radiopharmaceutical uptake. This influences cancer diagnosis and staging, as well as therapy response monitoring and radiotherapy planning. Finally, the possible impact of these developments on the European Association of Nuclear Medicine (EANM) guidelines and EANM Research Ltd. (EARL) accreditation for FDG-PET/CT tumor imaging is discussed.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0
- Record: found
- Abstract: found
- Article: not found
Fully 3-D PET reconstruction with system matrix derived from point source measurements.
- Record: found
- Abstract: found
- Article: not found
