- Record: found
- Abstract: found
- Article: found
Povidone-iodine for the management of oral mucositis during cancer therapy
Read this article at
Abstract
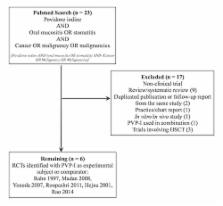
Oral mucositis (OM) is a common and often dose-limiting side effect of cancer therapy. Povidone iodine (PVP-I) formulations have been shown to decrease the incidence and severity of OM, but the relevance of these findings remains unclear. The objective of the present study was to review evidence for the use of PVP-I for OM management. An algorithm identified relevant articles published online, and a panel of experts with experience in the management of OM reviewed the findings. Six studies fulfilled the criteria for full review. Two studies provided evidence of moderate quality. Two of the studies with negative findings were confounded by the use of PVP-I concentrations that are too low to be efficacious. The remaining two studies were found to have design flaws. There exists reasonable evidence to support a recommendation for the use of PVP-I in the management of cancer therapy-related OM.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Updated clinical practice guidelines for the prevention and treatment of mucositis.
- Record: found
- Abstract: found
- Article: found
Practical use of povidone‐iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections
- Record: found
- Abstract: found
- Article: not found