- Record: found
- Abstract: found
- Article: found
Novel Markers to Delineate Murine M1 and M2 Macrophages
Read this article at
Abstract
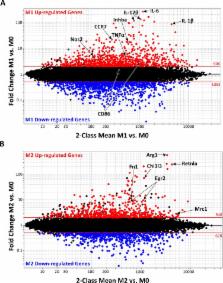
Classically (M1) and alternatively activated (M2) macrophages exhibit distinct phenotypes and functions. It has been difficult to dissect macrophage phenotypes in vivo, where a spectrum of macrophage phenotypes exists, and also in vitro, where low or non-selective M2 marker protein expression is observed. To provide a foundation for the complexity of in vivo macrophage phenotypes, we performed a comprehensive analysis of the transcriptional signature of murine M0, M1 and M2 macrophages and identified genes common or exclusive to either subset. We validated by real-time PCR an M1-exclusive pattern of expression for CD38, G-protein coupled receptor 18 (Gpr18) and Formyl peptide receptor 2 (Fpr2) whereas Early growth response protein 2 (Egr2) and c-Myc were M2-exclusive. We further confirmed these data by flow cytometry and show that M1 and M2 macrophages can be distinguished by their relative expression of CD38 and Egr2. Egr2 labeled more M2 macrophages (~70%) than the canonical M2 macrophage marker Arginase-1, which labels 24% of M2 macrophages. Conversely, CD38 labeled most (71%) in vitro M1 macrophages. In vivo, a similar CD38 + population greatly increased after LPS exposure. Overall, this work defines exclusive and common M1 and M2 signatures and provides novel and improved tools to distinguish M1 and M2 murine macrophages.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression.
- Record: found
- Abstract: found
- Article: not found
Cell type-specific involvement of RIG-I in antiviral response.
- Record: found
- Abstract: found
- Article: not found