- Record: found
- Abstract: found
- Article: found
Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
Read this article at
Abstract
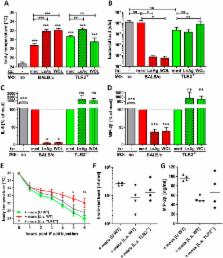
Helminths immunomodulate their hosts and induce a regulatory, anti-inflammatory milieu that prevents allergies and autoimmune diseases. Helminth immunomodulation may benefit sepsis outcome by preventing exacerbated inflammation and severe pathology, but the influence on bacterial clearance remains unclear. To address this, mice were chronically infected with the filarial nematode Litomosoides sigmodontis (L.s.) and the outcome of acute systemic inflammation caused by i.p. Escherichia coli injection was determined. L.s. infection significantly improved E. coli-induced hypothermia, bacterial clearance and sepsis survival and correlated with reduced concentrations of associated pro-inflammatory cytokines/chemokines and a less pronounced pro-inflammatory macrophage gene expression profile. Improved sepsis outcome in L.s.-infected animals was mediated by macrophages, but independent of the alternatively activated macrophage subset. Endosymbiotic Wolbachia bacteria that are present in most human pathogenic filariae, as well as L.s., signal via TLR2 and modulate macrophage function. Here, gene expression profiles of peritoneal macrophages from L.s.-infected mice revealed a downregulation of genes involved in TLR signaling, and pulsing of macrophages in vitro with L.s. extract reduced LPS-triggered activation. Subsequent transfer improved sepsis outcome in naïve mice in a Wolbachia- and TLR2-dependent manner. In vivo, phagocytosis was increased in macrophages from L.s.-infected wild type, but not TLR2-deficient animals. In association, L.s. infection neither improved bacterial clearance in TLR2-deficient animals nor ameliorated E. coli-induced hypothermia and sepsis survival. These results indicate that chronic L.s. infection has a dual beneficial effect on bacterial sepsis, reducing pro-inflammatory immune responses and improving bacterial control. Thus, helminths and their antigens may not only improve the outcome of autoimmune and allergic diseases, but may also present new therapeutic approaches for acute inflammatory diseases that do not impair bacterial control.
Author Summary
As the human immune system evolved in the presence of helminth infections, it is postulated that improved hygiene and subsequent loss of helminth infections and their immunomodulatory functions contributed to the sharp increase of autoimmune diseases and allergies over the last decades. Accordingly, helminth-induced anti-inflammatory, regulatory immune responses ameliorate allergy and autoimmune diseases and are likely to impact other immunological disorders including sepsis. Sepsis is an exacerbated, systemic inflammatory disease that occurs when pathogens cannot be locally confined and spread via the blood stream. Thus, efficient sepsis therapies should reduce excessive inflammation without impairing protective immune responses. In the present study we demonstrate that chronic filarial infection modulates macrophages to a less pro-inflammatory phenotype with improved phagocytic capacity. This immunomodulation reduces sepsis-induced inflammation and hypothermia and clears bacteria more efficiently thus improving sepsis survival. Moreover, we found that Wolbachia, the endosymbiotic bacteria of filariae, play a crucial role in triggering the correct macrophage response via TLR2. Thus, our observations suggest that helminths and helminth-derived antigens may not only present new treatment options for allergies and autoimmune diseases, but may also allow treatment of sepsis caused inflammation without impairing bacterial control.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Trichuris suis therapy in Crohn's disease.
- Record: found
- Abstract: found
- Article: not found
Suppression of allergic airway inflammation by helminth-induced regulatory T cells
- Record: found
- Abstract: found
- Article: not found