- Record: found
- Abstract: found
- Article: found
Exogenous bone marrow derived-putative endothelial progenitor cells attenuate ischemia reperfusion-induced vascular injury and renal fibrosis in mice dependent on pericytes
Read this article at
Abstract
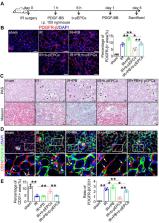
Rationale: Capillaries are composed of endothelial cells and the surrounding mural cells, pericytes. Microvascular repair after injury involves not only the proliferation of endothelial cells but also pericyte-based vessel stabilization. Exogenous bone marrow derived-putative endothelial progenitor cells (b-pEPCs) have the potential for vascular repair; however, their effect on vascular structure stabilization and pericyte-related pathobiological outcomes in the injured kidney has not been fully examined.
Methods: We applied ischemia-reperfusion (IR) to induce renal vascular injury and renal fibrosis in mice. Platelet-derived growth factor receptor β (PDGFR-β)-DTR-positive mice were generated to deplete pericytes, and exogenous b-pEPCs and the PDGFR-β ligand, PDGF chain B (PDGF-BB), were employed to explore the relationship among b-pEPCs, pericytes, vascular repair, and early renal fibrosis.
Results: Administration of b-pEPCs reduced IR-induced pericyte-endothelial detachment, pericyte proliferation, and myofibroblast transition via a paracrine mode, which preserved not only vascular stabilization but also ameliorated IR-initiated renal fibrosis. PDGF-BB upregulated the expression of PDGFR-β, exacerbated vascular abnormality, and pericyte-myofibroblast transition, which were ameliorated by b-pEPCs administration. The exogenous b-pEPCs and their culture medium (CM) induced vascular injury protection, and renal fibrosis was blocked by selective deletion of pericytes.
Conclusion: Exogenous b-pEPCs directly protect against IR-induced vascular injury and prevent renal fibrosis by inhibiting the activation of PDGFR-β-positive pericytes.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Pericytes: developmental, physiological, and pathological perspectives, problems, and promises.
- Record: found
- Abstract: found
- Article: not found
Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury.
- Record: found
- Abstract: found
- Article: not found