- Record: found
- Abstract: found
- Article: not found
An Advax-CpG55.2™ adjuvanted recombinant spike protein vaccine protects Cynomolgus macaques from a homologous SARS-CoV-2 virus challenge

Read this article at
Abstract
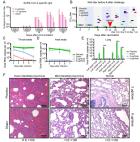
Traditional protein-based vaccine approaches to COVID-19 were overshadowed by the new mRNA and adenoviral vector vaccine approaches which were first to receive marketing authorization. The current study tested for the first time in repurposed aged (median 15.4 years) cynomolgus macaques, a novel Advax-CpG55.2™ adjuvanted recombinant extracellular domain spike protein trimer antigen combined with for immunogenicity, protection and safety. Nine animals received two intramuscular injections 10 days apart of recombinant spike protein (25 μg) with Advax-CpG55.2™ (10 mg/200 μg) adjuvant and the 5 controls received saline injections. Serum antibody levels were followed for 3 months and then the animals challenged with SARS-CoV-2 virus. Clinical signs, local reactions, body weight, food consumption and immunogenicity were monitored till termination at either day 3 or 7 post-infection. Two weeks after the second dose, 8/9 immunized macaques had high serum spike and receptor binding domain binding antibodies that were able to cross-neutralize Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and, to a lesser extent, Omicron variants (B.1.1.529 and sub-variants). However, antibody levels decayed over the next 3 months, with minimal neutralizing antibody detectable immediately prior to the challenge with a homologous Wuhan-like ancestral virus. Of the nine vaccinated animals, only one 18-year-old female sacrificed at d3 had low levels of recoverable lung virus, versus 100% of the control animals. Four of 5 (80%) control animals had positive lung staining for SARS-CoV-2 virus versus just 1 of 9 (11%) in the immunized group. The immunized animals exhibited better maintenance of appetite post-challenge. Although low pre-challenge, neutralizing antibody levels rebounded rapidly in immunized animals, post-challenge. This data supports the benefits of Advax-CpG adjuvanted recombinant spike protein vaccine in protecting against a homologous SARS-CoV-2 infection.
Related collections
Most cited references44

- Record: found
- Abstract: found
- Article: found
Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies

- Record: found
- Abstract: found
- Article: found
Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model
- Record: found
- Abstract: found
- Article: found
