- Record: found
- Abstract: found
- Article: found
Hemoadsorption in acute respiratory distress syndrome patients requiring venovenous extracorporeal membrane oxygenation: a systematic review
Read this article at
Abstract
Background
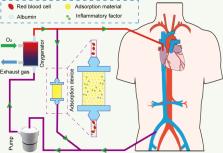
Venovenous extracorporeal membrane oxygenation (VV ECMO) has been widely used for severe acute respiratory distress syndrome (ARDS) in recent years. However, the role of hemoadsorption in ARDS patients requiring VV ECMO is unclear.
Methods
Therefore, we conducted a systematic review to describe the effect of hemoadsorption on outcomes of ARDS patients requiring VV ECMO and elucidate the risk factors for adverse outcomes. We conducted and reported a systematic literature review based on the principles derived from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The systematic review searched Embase, CINHAL, and Pubmed databases for studies on ARDS patients receiving hemoadsorption and VV ECMO. The demographic data, clinical data and biological data of the patients were collected.
Results
We ultimately included a total of 8 articles including 189 patients. We characterized the population both clinically and biologically. Our review showed most studies described reductions in inflammatory markers and fluid resuscitation drug dosage in ARDS patients with Coronavirus disease 2019 (COVID-19) or sepsis after hemoadsorption.
Conclusion
Because most of the studies have the characteristics of high heterogeneity, we could only draw very cautious conclusions that hemoadsorption therapy may enhance hemodynamic stability in ARDS patients with COVID-19 or sepsis receiving VV ECMO support. However, our results do not allow us to draw conclusions that hemoadsorption could reduce inflammation and mortality. Prospective randomized controlled studies with a larger sample size are needed in the future to verify the role of hemoadsorption in ARDS patients requiring VV ECMO.
Related collections
Most cited references61

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
- Record: found
- Abstract: found
- Article: found
Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China
- Record: found
- Abstract: found
- Article: found