- Record: found
- Abstract: found
- Article: found
Durable and dramatic response to checkpoint inhibition combined with COX‐2 inhibitor celecoxib in a patient with p16+ metastatic sinonasal undifferentiated carcinoma: A case study
Read this article at
Abstract
Background
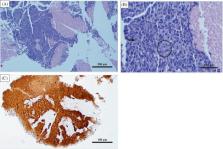
Sinonasal undifferentiated carcinoma (SNUC) is an exceedingly rare head and neck malignancy. No consensus exists on treatment for metastatic disease.
Case
A 56‐year‐old female was diagnosed with SNUC after endorsing sinus congestion, diplopia, and right orbital pain. Initially treated with surgery and radiation, she later developed significant metastatic disease. She demonstrated progression of her hepatic metastases under pembrolizumab therapy. However, the addition of ipilimumab and a COX‐2 inhibitor resulted in significant improvement in her lesions as well as an ongoing durable response. Her regimen was complicated by immune‐related adverse events successfully treated with steroids.
Related collections
Most cited references12

- Record: found
- Abstract: found
- Article: found
Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors
- Record: found
- Abstract: found
- Article: not found