- Record: found
- Abstract: found
- Article: found
Clinical outcomes of the Lotus Valve in patients with bicuspid aortic valve stenosis: An analysis from the RESPOND study
Read this article at
Abstract
Aims
Patients with bicuspid valves represent a challenging anatomical subgroup for transcatheter aortic valve implantation (TAVI). This analysis evaluated the clinical outcomes of the fully repositionable and retrievable Lotus Valve System in patients with bicuspid aortic valves enrolled in the RESPOND post‐market registry.
Methods and Results
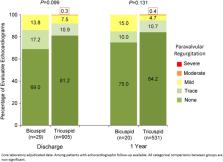
The prospective, open‐label RESPOND study enrolled 1,014 patients at 41 centers in Europe, New Zealand, and Latin America, 31 (3.1%) of whom had bicuspid aortic valves. The mean age in the bicuspid patient cohort was 76.4 years, 64.5% were male, and the baseline STS score was 6.0 ± 10.2. Procedural success was 100%, with no cases of malpositioning, valve migration, embolization, or valve‐in‐valve. Repositioning was attempted in 10 cases (32.3%). There was one death (3.2%) and one stroke (3.2%) at 30‐day follow‐up. Mean AV gradient was reduced from 48.7 ± 17.0 mmHg at baseline to 11.8 ± 5.1 mmHg at hospital discharge ( P < 0.001); mean effective orifice area (EOA) was increased from 0.6 ± 0.2 cm 2 to 1.7 ± 0.4 cm 2 ( P < 0.001). There were no cases of moderate or severe paravalvular leak (PVL) adjudicated by the core laboratory; four subjects (13.8%) had mild PVL, 5 (17.2%) had trace PVL. The rate of pacemaker (PM) implantation for PM‐naïve patients was 22.2% (6/27).
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document.
- Record: found
- Abstract: found
- Article: not found
A classification system for the bicuspid aortic valve from 304 surgical specimens.
- Record: found
- Abstract: found
- Article: not found