- Record: found
- Abstract: found
- Article: found
EGb761 Provides a Protective Effect against Aβ 1-42 Oligomer-Induced Cell Damage and Blood-Brain Barrier Disruption in an In Vitro bEnd.3 Endothelial Model
Read this article at
Abstract
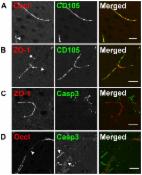
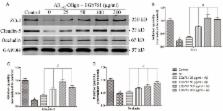
Alzheimer’s disease (AD) is the most common form of senile dementia which is characterized by abnormal amyloid beta (Aβ) accumulation and deposition in brain parenchyma and cerebral capillaries, and leads to blood-brain barrier (BBB) disruption. Despite great progress in understanding the etiology of AD, the underlying pathogenic mechanism of BBB damage is still unclear, and no effective treatment has been devised. The standard Ginkgo biloba extract EGb761 has been widely used as a potential cognitive enhancer for the treatment of AD. However, the cellular mechanism underlying the effect remain to be clarified. In this study, we employed an immortalized endothelial cell line (bEnd.3) and incubation of Aβ 1–42 oligomer, to mimic a monolayer BBB model under conditions found in the AD brain. We investigated the effect of EGb761 on BBB and found that Aβ 1–42 oligomer-induced cell injury, apoptosis, and generation of intracellular reactive oxygen species (ROS), were attenuated by treatment with EGb761. Moreover, treatment of the cells with EGb761 decreased BBB permeability and increased tight junction scaffold protein levels including ZO-1, Claudin-5 and Occludin. We also found that the Aβ 1–42 oligomer-induced upregulation of the receptor for advanced glycation end-products (RAGE), which mediates Aβ cytotoxicity and plays an essential role in AD progression, was significantly decreased by treatment with EGb761. To our knowledge, we provide the first direct in vitro evidence of an effect of EGb761 on the brain endothelium exposed to Aβ 1–42 oligomer, and on the expression of tight junction (TJ) scaffold proteins and RAGE. Our results provide a new insight into a possible mechanism of action of EGb761. This study provides a rational basis for the therapeutic application of EGb761 in the treatment of AD.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain.
- Record: found
- Abstract: found
- Article: not found