- Record: found
- Abstract: found
- Article: found
F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling

Read this article at
Abstract
During immune surveillance and inflammation, leukocytes exit the vasculature through transient openings in the endothelium without causing plasma leakage. However, the exact mechanisms behind this intriguing phenomenon are still unknown. Here we report that maintenance of endothelial barrier integrity during leukocyte diapedesis requires local endothelial RhoA cycling. Endothelial RhoA depletion in vitro or Rho inhibition in vivo provokes neutrophil-induced vascular leakage that manifests during the physical movement of neutrophils through the endothelial layer. Local RhoA activation initiates the formation of contractile F-actin structures that surround emigrating neutrophils. These structures that surround neutrophil-induced endothelial pores prevent plasma leakage through actomyosin-based pore confinement. Mechanistically, we found that the initiation of RhoA activity involves ICAM-1 and the Rho GEFs Ect2 and LARG. In addition, regulation of actomyosin-based endothelial pore confinement involves ROCK2b, but not ROCK1. Thus, endothelial cells assemble RhoA-controlled contractile F-actin structures around endothelial pores that prevent vascular leakage during leukocyte extravasation.
Abstract
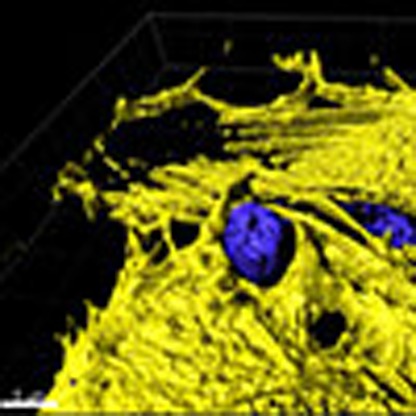 Endothelial cells can support leukocyte extravasation without causing vascular leakage,
but the exact mechanism underlying this process has not been fully elucidated. Here
the authors show that it is regulated through actomyosin-based endothelial pore confinement,
which requires local endothelial RhoA activation.
Endothelial cells can support leukocyte extravasation without causing vascular leakage,
but the exact mechanism underlying this process has not been fully elucidated. Here
the authors show that it is regulated through actomyosin-based endothelial pore confinement,
which requires local endothelial RhoA activation.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Spatiotemporal dynamics of RhoA activity in migrating cells.
- Record: found
- Abstract: found
- Article: not found
Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin.
- Record: found
- Abstract: not found
- Article: not found