- Record: found
- Abstract: found
- Article: found
Pathology after combined epicardial and endocardial ablation for ventricular tachycardia in a postmortem heart with hypertrophic cardiomyopathy
case-report
Kenzaburo Nakajima , MD
* ,
Koji Miyamoto , MD, PhD
*
,
* ,
Taka-aki Matsuyama , MD, PhD
† ,
Takashi Noda , MD, PhD
* ,
Hatsue Ishibashi-Ueda , MD, PhD
† ,
Kengo Kusano , MD, PhD
*
21 May 2015
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
KEY TEACHING POINTS
•
Lesion depth created by radiofrequency catheter ablation is smaller on the epicardium
with adipose tissue than on the endocardium.
•
It seems to be difficult to create transmural lesions even by combined epicardial
and endocardial ablation, especially in patients with thickened ventricular walls.
•
Some approaches such as needle, bipolar, and transcoronary ethanol ablation may be
more effective to create transmural lesions.
Ventricular arrhythmias in patients with nonischemic cardiomyopathy (NICM) often affect
patients’ prognosis. Ventricular tachycardias (VTs) arising from the electrical scar
area in hearts of NICM patients could become targets for radiofrequency catheter ablation
(RFCA) therapy.1, 2, 3 However, the efficacy of RFCA using the endocardial approach
is limited because of the deeper localization of the electrical scar from the intramural
to the epicardial side.1, 2, 3 Recently, combined endocardial and epicardial ablation
has been used to improve the RFCA outcomes for VT in patients with NICM. However,
elimination of the VTs is still challenging,1, 2, 3, 4, 5, 6 especially in patients
with hypertrophic cardiomyopathy (HCM).7, 8 In this case report, we evaluated the
pathologic features after combined epicardial and endocardial ablation in a postmortem
heart from a patient with HCM.
Case report
The patient was a 73-year-old man who was diagnosed with HCM at the age of 63 years.
The left ventricular chamber showed progressive dilation with contractile dysfunction
from the age of 67, and the heart was thought to be in an advanced dilated form. In
spite of the administration of β-blocker and amiodarone as concomitant medical therapy
for heart failure and nonsustained VT, the effect was insufficient, and he was implanted
with cardiac resynchronization therapy with defibrillator.
The patient was admitted to our hospital because of repetitive drug-resistant VT at
the age of 73 years. Echocardiography revealed an aneurysm at the apical portion of
the left ventricle (LV), which was accompanied by severe LV dysfunction (left ventricular
ejection fraction 21%) and asymmetric septal hypertrophy. On the day of admission,
the patient presented with VT storm, was intubated, and underwent assisted cardiopulmonary
support because the VTs were resistant to antiarrhythmic drugs and hemodynamically
unstable. Coronary angiography showed no significant stenosis in coronary arteries.
Under intravenous β-blocker administration, RFCA was performed to control the VT storm.
Electrophysiological examination and ablation procedure
Most of the clinical VTs in the 12-lead electrocardiogram (ECG) showed a right bundle
branch block pattern with slowed initial precordial QRS activation (Figure 1A). We
performed RFCA using the epicardial and endocardial approach because the origins of
some VTs were thought to be located on the epicardial side based on the 12-lead ECG
findings.
Steerable catheters were inserted from the right femoral vein and placed in the coronary
sinus and the ventricle of interest. The LV endocardium was accessed using the trans-septal
approach. Pericardial access was obtained through a subxiphoid and anterior puncture
under fluoroscopic guide.
We used a 3.5-mm open-irrigatedtip ablation catheter, Thermocool (Biosense Webster,
Johnson & Johnson, Diamond Bar, CA), which was also used for mapping. Bipolar voltage
maps of the endocardium and epicardium were constructed during sinus rhythm. Arrhythmogenic
areas, indicated by low-voltage zones (LVZs), which were defined as <1.5 mV at the
endocardium and <1.0 mV at the epicardium, were mainly located on the lateral to posterior
wall in the LV endocardium and throughout the entire LV in the epicardium (Figure
2A and B).
Six forms of VTs were induced by right ventricular pacing, although clinically documented
VTs did not appear (Figure 1B). We performed substrate-based RFCA, which targeted
the LVZs and abnormal electrograms such as delayed potentials, fragmented potentials,
and/or double potentials in the endocardium and epicardium. Open-irrigated radiofrequency
(RF) current was delivered for up to 60 seconds in power-controlled mode as follows:
power 30–45 W and irrigation 17–30 mL/min. Total application time of RFCA was about
86 minutes.
Clinical course after ablation
A VT that was not induced in the electrophysiological study emerged after the RFCA
session. The VT was clinically controlled by antiarrhythmic drugs (amiodarone, nifekalant,
and lidocaine). The patient, however, died from deterioration of heart failure 49
days after the procedure. A postmortem examination of the heart was performed.
Postmortem gross pathologic findings
The epicardium of the heart was mildly adhered to the pericardium around the apex
of the LV with a thin fibrinous exudate, and this was thought to be an inflammatory
reaction after the epicardial ablation procedure. There was no increase in pericardial
fluid volume.
The heart was significantly enlarged and weighed 820 g. There was rich epicardial
fat attached in the atrioventricular and interventricular groove, and it was also
widely distributed in the free wall of the LV that roughly corresponded to the voltage
map findings (Figure 2B and C). On the LV epicardial surface, RFCA results showed
discolored spotty lesions around the LV apex.
All 4 chambers of the heart were dilated along the long axis section through the heart.
The LV wall showed asymmetric hypertrophy as follows: 27 mm thickness in the ventricular
septum, and the papillary muscle was also hypertrophied (Figure 3A). The LV apical
wall was thinning with complex endocardial trabeculation, and it showed aneurysmal
dilation that was thought to be an arrhythmogenic source. The ablated lesions from
the endocardial approach were observed as blackish spots mainly located in and at
the border of the aneurysmal lesion. All coronary arteries showed no arteriosclerotic
stenosis.
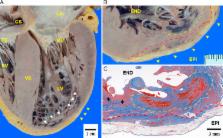
Ablated lesion beneath the epicardium and endocardium
Figure 3 shows a cross section of the ablated lesions at the border of the LV aneurysm,
where both the epicardial and endocardial approach ablation lesions were assessed.
The endocardial lesions were clearly defined and were deeper than the epicardial lesions.
The cloudy discolored areas were necrotic tissue caused by RF energy, and the adjacent
black-colored areas were lesions with hemorrhage caused by damage to intramural small
coronary arteries and capillaries. On the epicardial side, the LV apex around the
ablated lesions was diffusely covered by fatty tissue, and its thickness was up to
5 mm. The fatty layer was transmurally discolored; however, the change of the myocardium
beneath the epicardial fat was limited (Figure 3B).
Figure 3C shows the identical histologic sections to Figure 3B, and the original pathologic
fibrosis resulting from HCM diffusely extended mainly in the center and epicardial
side of the LV wall. The ablation scar reached a depth of 6 mm from the endocardial
surface at its deepest site, whereas the ablation scar from the epicardial surface
reached only 2 mm beyond the epicardial fat. Between the epicardial and endocardial
ablated lesions, there was approximately 1.5 mm of viable myocardial tissue with pathologic
fibrosis that escaped the RF energy at the border of the aneurysmal wall, and there
were no obvious transmural ablation lesions. In addition, the spared viable myocardium
was especially noted on the top of the trabeculi or bridging the myocardial bundles,
despite that the deeper sites were ablated.
The peripheral area of the ablated sites was replaced with mature fibrous tissue,
but the remaining necrotic tissue and hemorrhagic area were still observed at the
core of the ablated sites. This histologic discrepancy in the relatively long phase
after RF (approximately 6 weeks) may be attributed to the prolonged wound healing
process of the intramural hemorrhage and small vessel injuries.
9
Discussion
This case provided us with important information by the analysis of pathologic changes
after combined epicardial and endocardial ablation with open-irrigated catheter for
the first time in a human heart. We focused on the 2 major findings in this heart,
as described below.
The finding of voltage maps with larger LVZ on the epicardium than that on the endocardium
was roughly in accordance with the histopathology of the heart (Figure 3C and Supplemental
Figure, available online). We assumed that the LVZ on the epicardium, however, indicated
not only original pathologic fibrosis but also the fat on the epicardium. The pathologic
evaluation showed that transmural ablation could not be achieved in this case. Even
though new strategies such as local abnormal ventricular activity ablation or homogenization
of the scar area in electroanatomic mapping have been developed for unstable hemodynamic
VTs, efficacy of epicardial ablation is limited in cases like this, which might be
attributed to residual arrhythmic substrate, especially in NICM.10, 11 The saline
irrigation catheter produces deeper and larger ablation lesions in vivo compared with
a non–irrigation catheter.
12
However, despite the use of open-irrigated epicardial ablation, transmural ablation
lesions are found in only 55% of cases.
13
Some studies reported that the lesions made by epicardial ablations were affected
by the rich adipose tissues in the epicardium.
14
The degree of influence of the epicardial fat thickness on the RF delivery is controversial.
Van Huls van Taxis et al
15
reported the clear cut-off of thickness threshold to impair RF delivery as 7 mm. On
the other hand, Hong et al
16
reported that the epicardial fat could severely limit the lesion depth created by
RF energy, even in the case with the epicardial fat thickness of 2 mm.
16
In this case, ablated scars were not reached deeper from epicardial site than those
from endocardial site because of rich adipose tissues. The impaired RF delivery, which
was demonstrated by limited ablation lesions from the epicardial surface, might also
result from the catheter instability, weak contact force, and/or inadequate RF power
on the epicardium. The VT recurrence after RFCA might be also due to inability to
map and/or identify critical sites of VTs. We retrospectively examined the electrogram
response (R wave reduction of the electrogram) by RF delivery in this case, and compared
it between RF delivery from the epicardium and from the endocardium. In total, the
number of RF applications from the endocardium was 128 and that from the epicardium
was 109. The mean percentage of the R wave reduction by RF energy was significantly
lower in the epicardium than in the endocardium (59% ± 25% vs 69% ± 25%; P = .009).
In order to achieve transmural lesions, other approaches such as needle, bipolar,
and transcoronary ethanol ablation may be necessary, especially in cases with thickened
ventricular wall, as in this HCM patient.17, 18, 19
In this case, we could control VTs clinically with concomitant use of antiarrhythmic
drugs. Therefore, combined epicardial and endocardial ablation was useful to modify
the substrate for VTs in spite of the residual viable myocardial tissue between the
endocardium and epicardium in this case.
Conclusion
To our knowledge, this is the first case that shows important pathologic information
after combined epicardial and endocardial ablation using an open-irrigated catheter
in a human heart. This pathologic report showed the limitation of the epicardial RFCA
to deliver sufficient RF energy to the myocardium beyond the epicardial adipose tissue,
which resulted in residual arrhythmogenic substrate even after combined epicardial
and endocardial RFCA.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation.
C J McClelland, H Nakagawa, W Yamanashi … (1995)
- Record: found
- Abstract: found
- Article: not found
Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars.
G Couper, Peter Selwyn, Kyoko Soejima … (2004)
- Record: found
- Abstract: found
- Article: not found
Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits.
Jacob Koruth, Srinivas Dukkipati, Marc A Miller … (2012)