- Record: found
- Abstract: found
- Article: not found
Regulation of Lipolytic Response and Energy Balance by Melanocortin 2 Receptor Accessory Protein (MRAP) in Adipocytes
Read this article at
Abstract
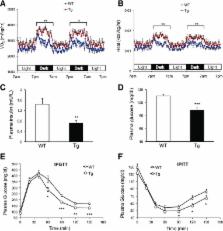
Melanocortin 2 receptor accessory protein (MRAP) is highly expressed in adrenal gland and adipose tissue. In adrenal cells, MRAP is essential for adrenocorticotropic hormone (ACTH)–induced activation of the cAMP/protein kinase A (PKA) pathway by melanocortin 2 receptor (MC2R), leading to glucocorticoid production and secretion. Although ACTH was known to stimulate PKA-dependent lipolysis, the functional involvement of MRAP in adipocyte metabolism remains incompletely defined. Herein, we found that knockdown or overexpression of MRAP in 3T3-L1 adipocytes reduced or increased ACTH-induced lipolysis, respectively. Moreover, an unbiased proteomics screen and coimmunoprecipitation analysis identified Gαs as a novel interacting partner of MRAP. An MRAP mutant disabled in Gαs association failed to augment the activation of PKA and lipolytic response to ACTH. Furthermore, compared with wild-type mice, transgenic mice (aP2-MRAP) overexpressing MRAP fat specifically exhibited increased lipolytic response to ACTH. When fed a high-fat diet (HFD), the transgenic mice displayed a significant decrease in the gain of adiposity and body weight as well as an improvement in glucose and insulin tolerance. These phenotypes were accompanied by increased adipose expression of genes for mitochondrial fatty acid oxidation and thermogenesis, and overall energy expenditure. Collectively, our data strongly suggest that MRAP plays a critical role in the regulation of ACTH-induced adipose lipolysis and whole-body energy balance.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase.
- Record: found
- Abstract: found
- Article: not found