- Record: found
- Abstract: found
- Article: found
Nitric Oxide and Biological Mediators in Pediatric Chronic Rhinosinusitis and Asthma
Read this article at
Abstract
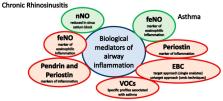
Background: In the context of the so-called unified airway theory, chronic rhinosinusitis (CRS) and asthma may coexist. The inflammation underlying these conditions can be studied through the aid of biomarkers. Main body: We described the main biological mediators that have been studied in pediatric CRS and asthma, and, according to the available literature, we reported their potential role in the diagnosis and management of these conditions. As for CRS, we discussed the studies that investigated nasal nitric oxide (nNO), pendrin, and periostin. As for asthma, we discussed the role of fractional exhaled nitric oxide (feNO), the role of periostin, and that of biological mediators measured in exhaled breath condensate (EBC) and exhaled air (volatile organic compounds, VOCs). Conclusion: Among non-invasive biomarkers, nNO seems the most informative in CRS and feNO in asthma. Other biological mediators seem promising, but further studies are needed before they can be applied in clinical practice.
Related collections
Most cited references132
- Record: found
- Abstract: found
- Article: not found
Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals.
- Record: found
- Abstract: found
- Article: not found