- Record: found
- Abstract: found
- Article: found
Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities

Read this article at
Abstract
Objective
To identify genes related to normal-pressure hydrocephalus (NPH) in one Japanese family with several members with NPH.
Methods
We performed whole-exome sequencing (WES) on a Japanese family with multiple individuals with NPH and identified a candidate gene. Then we generated knockout mouse using CRISPR/Cas9 to confirm the effect of the candidate gene on the pathogenesis of hydrocephalus.
Results
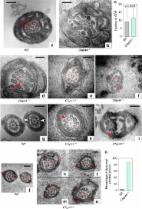
In WES, we identified a loss-of-function variant in CFAP43 that segregated with the disease. CFAP43 encoding cilia- and flagella-associated protein is preferentially expressed in the testis. Recent studies have revealed that mutations in this gene cause male infertility owing to morphologic abnormalities of sperm flagella. We knocked out mouse ortholog Cfap43 using CRISPR/Cas9 technology, resulting in Cfap43-deficient mice that exhibited a hydrocephalus phenotype with morphologic abnormality of motile cilia.
Related collections
Most cited references9

- Record: found
- Abstract: found
- Article: found
Human genetic variation database, a reference database of genetic variations in the Japanese population
- Record: found
- Abstract: found
- Article: not found
Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella.
- Record: found
- Abstract: found
- Article: found
Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human
Author and article information
Contributors
Journal
Affiliations
Author notes
Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article.
Author information
Article
Publisher ID: NEUROLOGY2018945816This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND), which permits downloading and sharing the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.