- Record: found
- Abstract: found
- Article: found
Picroside II Improves Severe Acute Pancreatitis-Induced Hepatocellular Injury in Rats by Affecting JAK2/STAT3 Phosphorylation Signaling

Read this article at
Abstract
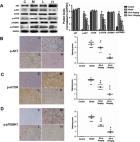
Picroside II is an important ingredient agent in Traditional Chinese medicine and hoped to reduce hepatocellular injury caused by severe acute pancreatitis (SAP). An SAP-induced hepatocellular injury model was established in rats by using pentobarbital sodium. 27 rats were divided into 3 groups: the sham group (SG), model group (MG), and Picroside groups (PG). SAP-induced hepatocellular injury was assessed using hematoxylin and eosin staining. We measured hepatocellular enzymes (amylase (AMY), alanine aminotransferase (ALT), and aspartate aminotransferase (AST)), oxidative stress factors (superoxidase dismutase (SOD) and malondialdehyde (MDA)), and inflammatory factors (tumor necrosis factor α (TNF- α), interleukin- (IL-) 6, and IL-10), apoptotic factors (BAX and cleaved caspase 3), and inflammatory signaling (Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), p-JAK2, and p-STAT3) in hepatocellular tissues. The SAP-induced hepatocellular injury model was successfully established. Picroside II treatment repaired hepatocellular injury by reducing the activities of AMY, ALT, and AST; reducing the levels of MDA, TNF- α, IL-1, IL-6, p-JAK2, p-STAT3, BAX, and cleaved caspase 3; and increasing the levels of SOD and IL-10. Picroside II exerted protective function for the SAP-induced hepatocellular injury model. Picroside II improved SAP-induced hepatocellular injury and antioxidant and anti-inflammatory properties by affecting JAK2/STAT3 phosphorylation signaling.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Bile acid metabolism and signaling in liver disease and therapy
- Record: found
- Abstract: found
- Article: found