- Record: found
- Abstract: found
- Article: found
Identification of a combined biomarker for malignant transformation in oral submucous fibrosis
Read this article at
Abstract
Background
Oral submucous fibrosis ( OSF) is a chronic progressive disease of the oral cavity that is considered a common potentially malignant disorder in South Asia. Areca nut chewing is the main etiological factor, but its carcinogenic mechanism has yet to be proven. The purpose of this study was to identify the useful biomarkers in predicting high‐risk patients with OSF.
Methods
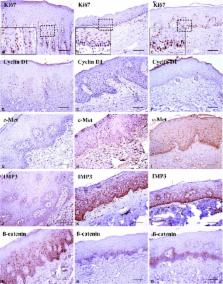
Thirty‐six cases of OSF and six cases of normal oral mucosa ( NOM) were used for this study. Immunohistochemical staining was performed for Ki67, cyclin D1, p16, p53, β‐catenin, c‐Jun, c‐Met, and insulin‐like growth factor II mRNA‐binding protein 3 ( IMP3). The expression patterns of NOM served as guidelines for the scoring system.
Results
The expression of Ki67, cyclin D1, c‐Met, IMP3, and β‐catenin showed a significant difference between OSF and NOM samples. The combined biomarkers of Ki67 and p16 showed significantly different expression between the transformation and non‐transformation groups. With discriminant analysis, we proposed a noble formula and cutoff value for predicting high‐risk patients with OSF.
Conclusion
The notable biomarkers in our present study were Ki67 and p16 showing significantly different expression levels between the transformation and non‐transformation groups. With the identification of high‐risk patients with OSF, we can expect to develop more intensive treatment modalities, leading to the reduction in cancer transformation rate from OSF.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms.
- Record: found
- Abstract: found
- Article: not found
Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory.
- Record: found
- Abstract: found
- Article: not found