- Record: found
- Abstract: found
- Article: found
Conflict of Interest Reporting by Authors Involved in Promotion of Off-Label Drug Use: An Analysis of Journal Disclosures
Read this article at
Abstract
Aaron Kesselheim and colleagues investigate conflict of interest disclosures in articles authored by physicians and scientists identified in whistleblower complaints alleging illegal off-label marketing by pharmaceutical companies.
Abstract
Background
Litigation documents reveal that pharmaceutical companies have paid physicians to promote off-label uses of their products through a number of different avenues. It is unknown whether physicians and scientists who have such conflicts of interest adequately disclose such relationships in the scientific publications they author.
Methods and Findings
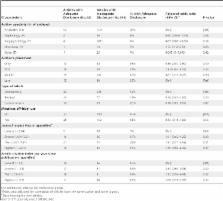
We collected whistleblower complaints alleging illegal off-label marketing from the US Department of Justice and other publicly available sources (date range: 1996–2010). We identified physicians and scientists described in the complaints as having financial relationships with defendant manufacturers, then searched Medline for articles they authored in the subsequent three years. We assessed disclosures made in articles related to the off-label use in question, determined the frequency of adequate disclosure statements, and analyzed characteristics of the authors (specialty, author position) and articles (type, connection to off-label use, journal impact factor, citation count/year). We identified 39 conflicted individuals in whistleblower complaints. They published 404 articles related to the drugs at issue in the whistleblower complaints, only 62 (15%) of which contained an adequate disclosure statement. Most articles had no disclosure (43%) or did not mention the pharmaceutical company (40%). Adequate disclosure rates varied significantly by article type, with commentaries less likely to have adequate disclosure compared to articles reporting original studies or trials (adjusted odds ratio [OR] = 0.10, 95%CI = 0.02–0.67, p = 0.02). Over half of the authors (22/39, 56%) made no adequate disclosures in their articles. However, four of six authors with ≥25 articles disclosed in about one-third of articles (range: 10/36–8/25 [28%–32%]).
Conclusions
One in seven authors identified in whistleblower complaints as involved in off-label marketing activities adequately disclosed their conflict of interest in subsequent journal publications. This is a much lower rate of adequate disclosure than has been identified in previous studies. The non-disclosure patterns suggest shortcomings with authors and the rigor of journal practices.
Please see later in the article for the Editors' Summary
Editor's Summary
Background
Off-label use of pharmaceuticals is the practice of prescribing a drug for a condition or age group, or in a dose or form of administration, that has not been specifically approved by a formal regulatory body, such as the US Food and Drug Administration (FDA). Off-label prescribing is common all over the world. In the US, although it is legal for doctors to prescribe drugs off-label and discuss such clinical uses with colleagues, it is illegal for pharmaceutical companies to directly promote off-label uses of any of their products. Revenue from off-label uses can be lucrative for drug companies and even surpass the income from approved uses. Therefore, many pharmaceutical companies have paid physicians and scientists to promote off-label use of their products as part of their marketing programs.
Why Was This Study Done?
Recently, a number of pharmaceutical companies have been investigated in the US for illegal marketing programs that promote off-label uses of their products and have had to pay billions of dollars in court settlements. As part of these investigations, doctors and scientists were identified who were paid by the companies to deliver lectures and conduct other activities to support off-label uses. When the same physicians and scientists also wrote articles about these drugs for medical journals, their financial relationships would have constituted clear conflicts of interest that should have been declared alongside the journal articles. So, in this study, the researchers identified such authors, examined their publications, and assessed the adequacy of conflict of interest disclosures made in these publications.
What Did the Researchers Do and Find?
The researchers used disclosed information from the US Department of Justice, media reports, and data from a non-governmental organization that tracks federal fraud actions, to find whistleblower complaints alleging illegal off-label promotion. Then they identified the doctors and scientists described in the complaints as having financial relationships with the defendant drug companies and searched Medline for articles authored by these experts in the subsequent three years. Using a four step approach, the researchers assessed the adequacy of conflict of interest disclosures made in articles relating to the off-label uses in question.
Using these methods, the researchers examined 26 complaints alleging illegal off-label promotion and identified the 91 doctors and scientists recorded as being involved in this practice. The researchers found 39 (43%) of these 91 experts had authored 404 related publications. In the complaints, these 39 experts were alleged to have engaged in 42 relationships with the relevant drug company: the most common activity was acting as a paid speaker (n = 26, 62%) but also writing reviews or articles on behalf of the company (n = 7), acting as consultants or advisory board members (n = 3), and receiving gifts/honoraria (n = 3), research support funds (n = 2), and educational support funds (n = 1). However, the researchers found that only 62 (15%) of the 404 related articles had adequate disclosures—43% (148) had no disclosure at all, 4% had statements denying any conflicts of interest, 40% had disclosures that did not mention the drug manufacturer, and 13% had disclosures that mentioned the manufacturer but inadequately conveyed the nature of the relationship between author and drug manufacturer reported in the complaint. The researchers also found that adequate disclosure rates varied significantly by article type, with commentaries significantly less likely to have adequate disclosure compared to articles reporting studies or trials.
What Do These Findings Mean?
These findings show the substantial deficiencies in the adequacy of conflict-of-interest disclosures made by authors who had been paid by pharmaceutical manufacturers as part of off-label marketing activities: only one in seven authors fully disclosed their conflict of interest in their published articles. This low figure is troubling and suggests that approaches to controlling the effects of conflicts of interest that rely on author candidness are inadequate and furthermore, journal practices are not robust enough and need to be improved. In the meantime, readers have no option but to interpret conflict of interest disclosures, particularly in relation to off-label uses, with caution.
Additional Information
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1001280.
-
The US FDA provides a guide on the use of off-label drugs
-
The US Agency for Healthcare Research and Quality offers a patient guide to off-label drugs
-
ProPublica offers a web-based tool to identify physicians who have financial relationships with certain pharmaceutical companies
-
Wikipedia has a good description of off-label drug use (note that Wikipedia is a free online encyclopedia that anyone can edit; available in several languages)
-
The Institute for Medicine as a Profession maintains a list of policies regulating physicians' financial relationships that are in place at US-based academic medical centers
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Pharmaceutical industry sponsorship and research outcome and quality: systematic review.
- Record: found
- Abstract: found
- Article: not found