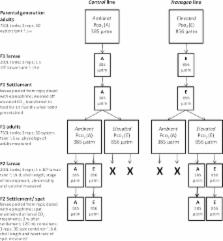
Introduction The sex ratio is a crucial demographic parameter important for population viability that is established by the processes of sex determination and differentiation. The sex determination mechanisms in vertebrates include genotypic sex determination (GSD), temperature-dependent sex determination (TSD) or a combination of both. In TSD, the temperature experienced during a particular time during early development, referred to as the thermosensitive period (TSP), irreversibly determines gonadal sex. TSD is well established in reptiles and fish [1]. Regardless of the sex determining system, in non-mammalian vertebrates the androgen-to-estrogen ratio determines whether a sexually undifferentiated gonad sexually differentiates into a testis or ovary. This sex steroid ratio depends of the activity of the enzyme aromatase, Cyp19a, the product of the cyp19a gene, which irreversibly converts androgens into estrogens. Further, in ectothermic vertebrates, the effects of environmental temperature on sex ratios are mediated by changes in cyp19a expression. Thus, in reptiles with TSD, exposure to female-promoting temperatures is invariably associated with gonadal cyp19a upregulation, whereas exposure to male-producing temperatures is associated with cyp19a suppression [2], [3]. In all fish species analyzed so far, more males are produced with increasing temperatures [4]. The masculinizing effects of high temperature are also invariably caused by an inhibition of cyp19a expression and enzymatic activity [5]–[7]. Thus, regardless of the animal group and the sex determining mechanism considered, cyp19a regulation is a key player in the sex ratio response to temperature in vertebrates. Unfortunately, the molecular mechanism by which temperature affects cyp19a has remained elusive [1], [8], and this is most important since identifying environmental cues and their perception and transduction mechanisms is a central focus of eco-devo research [9]. Gorelick [10] hypothesized that different methylation patterns of virtually identical sex chromosomes in species with TSD could be altered by small environmental changes, hence determining the sex of individuals. He also proposed that sex differences are initially determined by different patterns of methylation on nuclear DNA of females and males. Recently, reviewing the evidence gathered so far on DNA methylation of four steroidogenic enzymes, it has been postulated [11] that epigenetics are the missing link between genetics, the environment and endocrine functions. Furthermore, other recent studies [12] have shown epigenetic regulation not only of the enzymes involved in the steroidogenic pathway but also of some transcription factors and nuclear receptors related to steroid biosynthesis and action. In mammals, Cyp19 is expressed in a tissue-specific manner and regulated by different tissue-specific promoters [13]. Recently, epigenetic regulation of Cyp19 gene expression in mammals has been demonstrated in humans [14], cattle and sheep [15], [16], and buffalo [17]. The European sea bass (sb), Dicentrarchus labrax, is a gonochoristic GSD+TE species, which means that sex determination can be controlled by both genetic (GSD) and temperature effects (TE). Temperature and genetics contribute approximately equally to sex determination [18]–[20]. Importantly, both GSD+TE and “pure” TSD fish species have exactly the same response to high temperature: inhibition of cyp19a expression [4]. Thus, the sea bass is a perfectly suited model and indeed one of the best documented fish species in terms of sex ratio shifts in response to temperature [20]–[25]. Exposure to high temperatures (>17°C) during the TSP, which covers the period between fertilization to ∼60 days post fertilization (dpf), results in male-biased sex ratios (Figure S1) [20]. During sex differentiation, the primordial gonads, starting from a common primordium, can take two mutually exclusive different developmental pathways towards the formation of an ovary or a testis. Previous studies demonstrated that temperature effects in the European sea bass are more pronounced during the first half of the TSP, when fish are about 30 mm [20]. Interestingly, this not only occurs before morphological sex differentiation takes place (>150 dpf; ∼120 mm fish) but also even before the formation of the gonadal ridges at ∼35 dpf [26]. This demonstrates that the time when the temperature influence takes place is well before the actual differentiation period of the gonads into either the male or the female pathway. For that reason, we hypothesized the existence of an epigenetic mechanism activated by temperature, which could result in different levels of DNA methylation in the gonadal cyp19a promoter, which in turn would affect gene expression, estrogen synthesis and hence sex ratios. Results Methylation levels of the European sea bass gonadal cyp19a promoter are sex-specific and influenced by the temperatures experienced during early life To test our hypothesis, the previously characterized sea bass aromatase (sb cyp19a) promoter [27] was examined and the CpG dinucleotides ∼500 bp upstream of the transcription start site were selected (see Materials and Methods and Figure S2). First, gonadal methylation levels of the sb cyp19a promoter were determined using bisulfite sequencing in one-year-old sea bass males and females (family 1) exposed to two different temperature regimes during the first 60 days of life (high temperature, HT group, and low temperature, LT group; see Materials and Methods and Figure S3). A two-way ANOVA indicated significant differences in average DNA methylation levels of the sb cyp19a promoter in one year old sea bass (∼160 mm length; ∼73 g weight) according to sex (F = 118.2; P = 0.001) and temperature treatment (F = 14.6; P = 0.000), but without interaction between the two variables (P = 0.703). Results showed that, overall, males had twice as much sb cyp19a promoter DNA methylation levels as females (mean ± S.E.M.: 81.15±2.54% vs. 45.5±3.47%; two-tailed Student's t-test; t = −9.591, P = 0.000; Figure S4). Furthermore, sex-related differences were also clearly evident by distinct frequency distributions, with values in the range 12.9–72.8% for females and 71.4–97.1% for males, with a threshold value of 67% (Figure S4). The most important finding, however, was that exposure to high temperature increased gonadal sb cyp19a methylation levels in females from 37.1±3.45 to 53.9±3.49% (two-tailed t-test; t = 3.186, P = 0.005), and from 77.0±1.81 to 85.3±3.28% in males (two-tailed t-test, t = 2.056, P = 0.062; Figure 1). 10.1371/journal.pgen.1002447.g001 Figure 1 Resulting differences in sb cyp19a promoter methylation according to sex and temperature treatment. Typical methylation patterns of sea bass gonadal aromatase promoter of one-year-old sea bass females and males that were reared at low and high temperature during early development, as observed in this study. One fish for each sex and temperature combination representative of the level of methylation is shown. Numbers with a plus or minus sign indicate CpG positions with respect to the transcription starting site. Open and filled circles denote unmethylated or methylated positions, respectively. Ten clones per fish were analyzed. Average methylation was calculated specifically for each CpG position (number below each column). The number inside the bar indicates sample size. Results as mean ± SEM. Groups with different letters are significantly different (P 5. A, Frequency of the methylation pattern consisting of all seven positions methylated. B, Frequency of the methylation pattern with the 5′first position (−431) unmethylated and the remaining six methylated. C, Frequency of the methylation pattern with the last position (+60) unmethylated and the remaining six methylated. D, Frequency of the methylation pattern with all positions unmethylated. Abbreviations: Females at Low Temperature (FLT); Females at High Temperature (FHT); Males at Low Temperature (MLT); Males at high temperature (MHT). The level of significance is indicated for each case. To examine the origin of these sex- and temperature-related differences, DNA methylation levels of the gonadal sb cyp19a promoter were assessed in much younger fish (94.8±0.08 mm) of an unrelated batch (family 2), in which biopsy confirmed that they were not sexually differentiated (sex differentiation in the European sea bass starts when fish are in the range ∼80–120 mm). The same temperature treatments were applied as explained above (high temperature, HT group and low temperature, LT group; see Materials and Methods and Figure S3). Since phenotypic sex was unknown in those individuals, a two-step unrestricted cluster analysis of cyp19a mRNA levels was used to classify fish as presumptive future males (low cyp19a mRNA levels) and presumptive future females (high cyp19a mRNA levels) within each temperature treatment (Figure 3), a procedure we had previously demonstrated to be reliable [21]. A two-way ANOVA analysis indicated significant differences in average sb cyp19a gene expression according to sex (F = 110.1; P = 0.000) but not to temperature treatment (F = 1.2; P = 0.277), but with interaction between the two variables (P = 0.013). Two-tailed t-tests also showed differences between cyp19a expression levels (RQ) of LT males vs. LT females (t = 8.427; P = 0.000), HT males vs. HT females (t = 6.251; P = 0.000), HT females vs. LT females (t = −2.516; P = 0.024), but no differences between the RQ values of HT males vs. LT males (P = 0.243) (Figure 3). Furthermore, methylation levels were also examined in 12 individuals who happened to be classified as presumptive females by the cluster analysis. In the HT group, cyp19a promoter DNA methylation levels were 79.0±3.43% (mean ± SEM, n = 3), with a coefficient of variation of 7.5% and a range of 77.2–85.7%. In the LT group, sb cyp19a promoter DNA methylation levels were 80.6±2.55% (two-tailed t-test, t = −0.373, P = 0.717), with a coefficient of variation of 9.5%. However, in one of the nine fish examined the value of cyp19a promoter methylation was 61.9%, i.e., below the threshold level of 67% calculated with the 95% confidence interval of DNA methylation levels observed in adult males vs. adult females (Figure S4), further suggesting that this fish was most likely a female. 10.1371/journal.pgen.1002447.g003 Figure 3 Gonadal aromatase (cyp19a) expression in sexually undifferentiated sea bass. Gonadal aromatase expression was assessed by real time RT-PCR and used as a marker to classify fish into presumptive females (red circles) or presumptive males (blue circles) for the two thermal treatments, low and high temperature. Individual sample points are presented along with the median. Groups with different letters are significantly different (P 0.05). High temperatures experienced by the European sea bass during early life are able to masculinize populations by decreasing cyp19a mRNA expression levels in the female gonads When histologically sexed at about one year of age, the percentage of females in the HT group (family 1) was 56.0±11.3% (n = 40), a 15% decrease when compared to the 71.0±3.5% (n = 40) value of the LT group (Chi square = 7.28; P = 0.01). On the other hand, cyp19a mRNA expression levels in HT females was significantly lower than in LT females (t-test; F = 0.024; P = 0.003; Figure 5A). In addition, there was a significant inverse relationship between cyp19a expression and methylation levels in these one-year old females (r2 = 0.29; F = 7.84; P = 0.01) (Figure 5B). 10.1371/journal.pgen.1002447.g005 Figure 5 Effects of temperature on sb cyp19a promoter methylation levels and correlation with gonadal cyp19a gene expression. A, Female sb cyp19a expression assessed by Q-PCR; data as mean ± S.E.M (see main text for details). Groups with different letters are significantly different (P 0.05). Methylation levels of specific CpGs within the sb cyp19a promoter are sex- and temperature-dependent Analysis of Molecular Variance (AMOVA) was used to determine if the different CpGs positions found in the sb cyp19a promoter were differentially methylated between male and female gonads or between the gonads of animals of the same sex but reared at different temperatures. Results revealed that particular CpGs were differentially methylated according to sex and/or temperature (Figure 5C and 5D). Significant differences in all positions were detected between LT males and LT females (AMOVA, Fst = 0.361, P = 0.000) (Table 1). However, only CpG positions −431 and −13 showed significant differences (AMOVA, Fst = 0.052, P = 0.014) when LT females were compared with HT females, with position −13 presenting the highest significant differences (Fst = 0.083, P = 0.031 and Fst = 0.148, P = 0.005, respectively; Figure 5C and Table 1). 10.1371/journal.pgen.1002447.t001 Table 1 Effects of high temperature on DNA methylation of the sea bass gonadal aromatase promoter at different loci. LT Males versus LT Females LT Females versus HT Females CpG position Fst P Fst P −431 0.362 0.000 0.083 0.031 −56 0.219 0.000 −0.007 0.535 −49 0.404 0.000 0.009 0.325 −33 0.363 0.000 0.061 0.058 −13 0.575 0.000 0.148 0.005 +9 0.318 0.000 0.019 0.241 +60 0.168 0.005 0.045 0.104 For each CpG, differences between males and females exposed at low temperature (LT), and between females reared at LT and high temperature (HT) are reported. Statistically significant differences are highlighted in bold face. Abbreviations: Fst, Fixation index of population differentiation; P, significance level. Methylation of the sb cyp19a promoter blocks SF-1 and Foxl2 stimulated cyp19a expression in vitro In a previous study [27], we characterized the sb cyp19a promoter using bioinformatic tools (MatInspector) and gel shift assays and identified putative transcription binding sites. From that study it was found that the CpG in position −431 of this promoter is located near a putative binding site for a transcription factor of the Fox family and that the CpG in position −13 is located near a putative binding site for a Sox family transcription factor (Figure S2). Furthermore, previous studies with other fish species have shown that co-transfection of sb cyp19a promoter constructs with either Foxl2, SF-1 or simultaneous co-transfection with both of these potent transcriptional activators of cyp19a significantly increased luciferase activity [28], [29]. Based on these previous findings, the sb cyp19a promoter activation was determined under methylated and control conditions by a luciferase reporter assay. As expected, SF-1 and Foxl2 were each capable of activating sb cyp19a expression in vitro. When combined, still higher expression was observed (Figure 7). Remarkably, induced hypermethylation of the sb cyp19a promoter completely suppressed promoter transcription stimulation in vitro by Foxl2 and SF-1 alone (two tailed t- test, P 18 mm), juveniles were reared in 650 l fiberglass tanks under simulated natural photoperiod and fed ad libitum with pelleted food of the appropriate size. Temperature treatments Eggs were incubated at 14–15°C, the natural temperature for sea bass spawning and fertilization during winter and early spring in the western Mediterranean. For this study, a total of 3 different egg batches originated from different parents (i.e., 3 different families) were used. Hatching occurred 3–4 days post fertilization (dpf). At this point, fish were separated into two groups. One group was reared at 15°C throughout the thermosensitive period (TSP) until 60 dpf (“low temperature”, LT or control group). Then, temperature was increased to 21°C at a rate of 0.5°C·day−1 and left to follow the natural fluctuations until the end of the study, when fish were about one year old (330 dpf). Twenty-one degrees Celsius is the standard temperature for ensuring adequate growth in juvenile sea bass rearing. The other group of fish was reared at 15°C until 10 dpf and then switched to 21°C (Figure S3). Thus, fish in this group were exposed to artificially higher temperature (HT) essentially during the entire TSP, which promotes male development. Each temperature treatment was carried out in duplicate. In summary, while the thermal regimen of the LT group is similar to the one experienced by sea bass in the wild, the thermal regimen of the HT group typically results in the masculinization of about one-half of the genotypic females into phenotypic males [19], [20]. For these experiments, two different sea bass families (families 1 and 2) were used: sb cyp19a and β-actin promoter methylation was studied in brain and gonads and cyp19a gene expression in gonads of sexually differentiated, one-year-old animals (family 1), and sb cyp19a promoter methylation and gene expression was studied in the gonads of sexually undifferentiated animals (family 2). Estrogen treatments An additional group of fish subjected to the same LT regime as described above was fed with a diet containing estradiol-17β (E2) at a concentration of 10 mg/kg of food. Treatment was carried out well past the TSP, from 90–150 dpf, coinciding with the labile period for steroid treatment [19]. This experiment was carried out to test whether E2 treatment was capable of affecting sb cyp19a promoter methylation, and hence, in order to maximize the expected feminizing effect of E2, in this case a family giving low numbers of females even at LT was used (family 3). At about one year of age, fish (n = 40) were sexed and DNA methylation levels were determined as explained below. Ethics statement In all cases, fish were treated in agreement with the European Convention for the Protection of Animals used for Experimental and Scientific Purposes (ETS N° 123, 01/01/91). Sampling and gonadal histology At 330 dpf, i.e., long past the thermal regimes, fish were sacrificed and gonadal samples were collected (n = 40 fish per treatment). From each fish (∼159 mm and ∼73 g), one gonad was processed for histological identification of sex. Gonads were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, cut at 7 µm thickness and stained with haematoxylin-eosin. The other gonad was snap-frozen in liquid nitrogen and stored at −80°C until further analysis to determine methylation levels and gene expression. For sexually undifferentiated fish (family 2; 94.8±0.08 mm), 20 fish per group were collected from the LT and HT treatment groups. Due to tissue amount limitations, the right gonad was used to obtain DNA and the left gonad to obtain RNA. Sex was identified based on cyp19a mRNA levels as described in Blázquez and collaborators [41] and in the Statistical analysis section below. Methylation levels measured by bisulphite-mediated genomic sequencing The gonadal sb cyp19a [27] and β-actin promoters [54] were examined to identify CpG dinucleotides that could be differentially methylated. For the sb cyp19a promoter (Figure S2), genomic DNA was obtained in the case of sexually differentiated fish from the gonads of 8–15 fish or the brains of 3–5 fish, depending on temperature and sex. For the β-actin promoter, brains and gonads from 3–5 animals were used depending also on temperature and sex. In the case of sexually undifferentiated fish, a total of 12 animals were used for sb cyp19a promoter methylation analysis. The DNA samples from each animal were individually processed and subjected to sodium bisulphite-mediated sequencing as described by Widschwendter and collaborators (2000) [55]. The targeted portion of the promoter was amplified from the bisulphite-modified DNA with two rounds of PCR by use of nested primers specific to the bisulphite-modified sequence of this region. For the sb cyp19a promoter the primers were as follows: External Forward, ATTGGTAGTTTAATGGAGGAATTT; External Reverse, AATCCCACTACAATAACATTTAAAAAC; Nested Forward, GAGGAATTTGGGAGGAATTATAAATAT; Nested Reversed, CCAAATCTACCACTATAATATCCAAAC. The primers for the β-actin promoter were as follows: External Forward, AATTTATAATTTTGGTTGGTAGTAA; External Reverse,CAAAATCTTACCTTAAAAATATATCTAC; Nested Forward, TATAATTTTGGTTGGTAGTAATTGG; Nested Reverse, CATTCACAAACCTCAACACTAACC. A hot start polymerase (Qiagen) was used in both external and nested PCR. For the sb cyp19a promoter, external PCR consisted in 5 min at 94°C; 5 cycles of 1 min at 94°C, 2 min at 55°C, 3 min at 72°C; 25 cycles of 30 s at 94°C, 2 min at 50°C, 1 min and 30 s at 72°C and a final extension of 7 min at 72°C. Subsequently, nested PCR consisted in 5 min at 94°C; 30 cycles of 30 s at 94°C, 30 s at 56°C, 30 s at 72°C and a final extension of 7 min at 72°C. For the β-actin promoter, external PCR consisted in 5 min at 94°C; 5 cycles of 1 min at 94°C, 2 min at 54°C, 3 min at 68°C; 25 cycles of 30 s at 94°C, 2 min at 50°C, 1 min and 30 s at 68°C and a final extension of 7 min at 72°C. Subsequently, nested PCR consisted in 3 min at 94°C; 30 cycles of 30 s at 94°C, 30 s at 53°C, 30 s at 68°C and a final extension of 7 min at 72°C. PCR products were separated by gel electrophoresis and gel bands were purified by Purelink Quick Gel Extraction (Invitrogen). Gel purified bands were cloned into the pCR4-TOPO vector and transformed into E. coli Topo10 chemically competent cells (Invitrogen) in the case of the sb cyp19a promoter, or into pGEM-T Easy vector (Promega) and transformed into E. coli JM109 competent cells (Invitrogen) in the case of the β-actin promoter. Then 7–10 individual clones per each fish were sequenced each in both directions and used to evaluate the seven (cyp19a) or 25 (β-actin) CpG dinucleotide positions present in the promoter region. Average methylation levels per position and fish were computed. In summary, in this study a total of 76 different animals were used. DNA methylation levels were determined, always on an individual fish basis (no pools were used), only in the gonads in some fish, whereas in others it was determined both in the gonads and also in the brain, and, as stated above, 7–10 clones per fish were sequenced in both directions. The total amount of sequenced clones in this study was ∼1100. The efficiency of the bisulfite conversion step was evaluated by using the Bisulfite sequencing Data Presentation and Compilation (BDPC) online software (available at: http://biochem.jacobs-university.de/BDPC/index.php) [56]. The conversion rates of C, which are not in the context of a CpG, were determined in different number of clones and in all tested tissues. For the sb cyp19a promoter, the mean percentage of converted Cs was 97.98±0.9% (calculated in a subset of 35 PCR reactions) and for β-actin promoter, the mean percentage was 97.68±0.41% (calculated from a subset of 71 PCR reactions). Since ten clones per fish were used to determine sb cyp19a promoter methylation, the maximum number of different methylation patterns (or epialleles) per fish that could be observed was 10, i.e., each clone has a different methylation pattern. Thus, for each fish from each one of the four groups the actual observed number of different cyp19a promoter methylation patterns was determined in order to check for possible PCR bias. sb cyp19a gene expression measurement by real-time RT–PCR Total RNA was isolated from ovaries of 8 females from the HT group and 14 females from the LT group from sexually differentiated one-year-old sea bass, and 16 gonads from the HT and 16 from the LT group in the case of sexually undifferentiated or differentiating animals. Gonad tissue was homogenized with 0.5 ml of trizol and total RNA was extracted with chlorophorm, precipitated with isopropanol and washed with 75% ethanol. Pellets were suspended in 25 µl DEPC-water and stored at −80°C. One microgram of total RNA was reverse transcribed into cDNA using Superscript II (Invitrogen) and 250 ng of random hexamer primers (pdN6) following the manufacturer's instructions. Real-time PCR reactions were carried out to determine sb cyp19a gene expression. The endogenous reference gene used was 18S (validated in a previous study by our laboratory [57]). The real time RT-PCR reaction was carried out with the SYBR Green chemistry (Power SYBR Green PCR Master Mix; Applied Biosystems). The primers were: ovaro RT-F1: AGACAGCAGCCCAGGAGTTG and ovaro RT-R1: TGCAGTGAAGTTGATGTCCAGTT. PCR reactions contained 1X SYBR green master mix (Applied Biosystems), 10 pmol of each primer and 1 µl of the RT reaction. Samples were run in duplicate in optically clear 384-well plates. Cycling parameters were: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Finally, a temperature-determining dissociation step was performed at 95°C for 15 s, 60°C for 15 s and 95°C for 15 s at the end of the amplification phase. Real-time RT-PCR data were collected by SDS 2.3 and RQ Manager 1.2 software and relative quantity (RQ) values were estimated for each reaction replicate. Specifically, the female with the lowest level of aromatase expression (i.e., highest ΔCt) was assigned as the calibration sample to calculate ΔΔCt and RQ values. cyp19a promoter activation by luciferase reporter assay Plasmid construction Genomic DNA was used to clone the sb cyp19a promoter by PCR. The primers used were: AroALucF, GCAGAGGTAGGAACACAGTTCA and AroALucR, CATTTGGGGACGTGGAGA. To each 5′-end primer sequence a restriction site for XhoI enzyme was added. Promoter sequence was obtained using Easy-A High-fidelity PCR cloning polymerase (Stratagene) and PCR conditions were: 2 min at 95°C; 25 cycles of 30 s at 95°C, 30 s at 68°C, 2 min and 30 s at 72°C and a final extension of 5 min at 72°C. The amplified promoter fragment was gel purified and cloned into pGEM-T (Promega). The sb cyp19a promoter was subsequently digested with XhoI enzyme from pGEM-T vector and cloned into XhoI digested pGL3 Basic Vector (Promega). In vitro methylation of plasmid reporters pGL3-cyp19a plasmids were cytosine-methylated using SssI methylase according to the manufacturer's instructions (New England BioLabs). SssI methylation, which methylates all cytosine residues within the double-stranded dinucleotide recognition sequence (5′-CG-3′), was performed with 10 mM Tris, pH 7.9, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, and 160 µM S-adenosylmethionine at 37°C for 1 h. After the methylation, reaction plasmids were purified by phenol extraction. Successful vector methylation was checked by analyzing band patterns on gel electrophoresis after digestion of the purified plasmids with the McrBC enzyme, which digests only methylated DNA, according to the manufacturer's instructions (New England BioLabs). Fully methylated plasmids were utilized for transient transfection assays. Transfection and luciferase reporter gene assay Human embryonic kidney 293T (HEK 293T) cells were transfected using the calcium phosphate coprecipitation method [58] with pGL3-cyp19a methylated and unmethylated promoter vectors. Transcription factors SF1 and Foxl2 were cotransfected with the sb cyp19a promoter to activate promoter luciferase activity. Transfected methylated and unmethylated groups were as follows: 1) 500 ng of plasmid β-galactosidase (Ctrl); 2) 2.5 µg of sb cyp19a promoter cloned into pGL3-basic luciferase reporter plasmid (cyp19a); 3) 2.5 µg of cyp19a and 250 ng of tilapia SF1 transcription factor cloned into pCDNA3.1 expression plasmid (cyp19a+SF1); 4) 2.5 µg of cyp19a and 250 ng of tilapia Foxl2 transcription factor cloned into pCDNA3.1 expression plasmid (cyp19a+Foxl2); 5) 2.5 µg of cyp19a, 250 ng of tilapia SF1-pCDNA3.1 and 250 ng of tilapia Foxl2-pCDNA3.1 (cyp19a+SF1+Foxl2). Five hundred nanograms of plasmid β-galactosidase were co-transfected in all cases as an internal control of transfection efficiency. All transfections were carried out at least in duplicate. Cells were incubated for 16 h with precipitated vectors-CaPO4. Forty-eight hours after transfection, cells were washed with PBS and lysed in 400 µl luciferase lysis buffer. Lysate was analyzed for Luc activity using the luciferase assay system (Promega) in an Orion Microplate luminometer (Berthold). β-galactosidase activity was used to normalize the results. Statistical analyses Data reported as proportions (sex ratios and methylation levels) were always arcsin square root transformed before any statistical analysis. Likewise, all RQ expression data were log-transformed to ensure normality. A two-way ANOVA was used to investigate differences in sb cyp19a or β-actin promoter DNA methylation levels (dependent variable) considering sex and temperature as the two independent factors. Post hoc multiple comparisons were carried out with Tukey's multiple range test with the Statgraphics v16 or SPSS v19 software. One-year-old fish were histologically sexed. Younger, sexually-undifferentiated fish were sexed based on a previous study from our laboratory that demonstrated that mRNA levels of cyp19a can be used as an early marker of phenotypic sex in the European sea bass [41]. In this case, a two-step cluster analysis (SPSS) was used to classify individuals as presumptive females and males based on the gonadal cyp19a mRNA levels (RQ). Afterwards, a two-way ANOVA was used to investigate differences between temperature and phenotypic sex as explained above. In addition, a two-tailed Student's t-test was used to check for differences in RQ between the following pairs: presumptive males and females at LT; presumptive males and females at HT; presumptive females at LT and HT; and presumptive males at LT and HT. Two-tailed Student's t-test was also used to analyze differential cyp19a expression levels among one-year old females of each temperature treatment and also to detect differences between pGL3-cyp19a methylated and unmethylated promoter vectors in the transfection assay. To check for differences in methylation levels in specific CpG positions of the sb cyp19a promoter, a hierarchical population analysis was carried out. First, sequences were trimmed to seven nucleotides in length, one corresponding to each CpG analyzed, with two possible variants for each nucleotide: C if methylated and T if unmethylated. Then, all sequences from the same individual were considered as one population, with size equivalent to the number of sequences analyzed for each individual [59]. The four classes (i.e., treatments, LT and HT males, and LT and HT females) were considered as groups of populations. The hierarchical analysis of the molecular variance, AMOVA [59], was used to test for possible genetic differentiation among classes. When less than 5% of the 10000 pseudo-replicates presented higher genetic variance than one estimated by chance, then the genetic structure was considered not significant. AMOVA also calculated the correlation measure fixation index of population differentiation (Fst). In all cases differences were considered statistically different when P<0.05. Supporting Information Figure S1 Pattern of observed sex ratio responses to temperature in the European sea bass. Resulting number of phenotypic females as a function of the rearing temperature during the thermosensitive period (up to ∼60 days post fertilization). Data from different studies [20] with different families and expressed as mean ± S.E.M. of n ∼5 trials for each temperature. The 13–17°C range corresponds to the natural range of temperatures during sea bass spawning and larval development, and explains why at these temperatures sex ratios approach the 1∶1 Fisherian sex ratio. In contrast, 21–22°C is the commonly used temperature for larval rearing during sea bass farming. Thus, an increase of only 4°C is able to result in strongly male-biased sex ratios. (PPT) Click here for additional data file. Figure S2 Diagram of the sea bass (sb) gonadal aromatase (cyp19a) promoter region analyzed in this study. Genomic DNA was extracted from the gonads of individual fish. Restriction enzyme digestion (BglII and DraII) outside the region of interest was used to obtain a smaller and linearized fragment of the promoter. After bisulphite treatment, external and nested PCR was carried out to amplify a 598 bp PCR fragment. Inside this region, putative transcription factors binding sites as well as CpG dinucleotide localizations (lollipops) are shown, indicating their nucleotide position with respect to the transcription start site. Transcription and translation starting sites are symbolized with an arrow and an asterisk, respectively. Abbreviations for binding sites: Fox, forkhead transcription factor; Sox, Sry-related transcription factor; Are, androgen response element; SF1, steroidogenic factor-1; Ppar, peroxisome proliferation activated receptor; Cre, cAMP response element; TATA, TATA box. (PPT) Click here for additional data file. Figure S3 Thermal protocols applied in the present study. The experimental groups (carried out in duplicate) were: Low temperature, LT, 15°C from 0–60 dpf, thereafter following the natural fluctuation; and high temperature, HT, 15°C from 0–10 dpf, then at 21°C throughout the thermosensitive period (TSP). The TSP and the sex differentiation period are indicated (with a dashed line and a line between arrows, respectively) in relation to the thermal regimens. The dashing pattern indicates that the effects of temperature are more evident shortly after fertilization and progressively wear out. The major events related to gonad formation and sex differentiation are also indicated. (PPT) Click here for additional data file. Figure S4 Frequency distribution of average cyp19a promoter DNA methylation levels in relation to phenotypic sex in the European sea bass. Sex-specific differences in cyp19a promoter methylation in adult sea bass gonads. The dashed line indicates the methylation threshold (67%) calculated with the 95% confidence interval, which separates typical sea bass female and male cyp19a methylation levels. (PPT) Click here for additional data file. Figure S5 Absolute frequency of the 58 methylation patterns observed (out of the 128 theoretically possible methylation patterns) according to sex and temperature treatment. Yellow circle, females at low temperature (FLT); red square, females at high temperature (FHT); light blue square, males at low temperature (MLT); dark blue triangle, males at high temperature (MHT). (PPT) Click here for additional data file. Figure S6 Number of different methylation patterns observed. A, distributed according to sex and temperature treatments, and B, in relation to levels of cyp19a promoter methylation. Boxed numbers in panel A are the average number of observed methylation patterns in each group. Yellow circle, females at low temperature (FLT); red square, females at high temperature (FHT); light blue square, males at low temperature (MLT); dark blue triangle, males at high temperature (MHT). (PPT) Click here for additional data file.