- Record: found
- Abstract: found
- Article: not found
Metabotropic Glutamate Receptor 1 acts as a Dependence Receptor Creating a Requirement for Glutamate to Sustain the Viability and Growth of Human Melanomas
Read this article at
Abstract
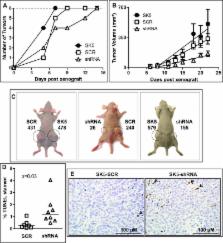
Metabotropic glutamate 1 (mGlu) receptor has been proposed as a target for the treatment of metastatic melanoma. Studies have demonstrated that inhibiting the release of glutamate (the natural ligand of mGlu1 receptors), results in a decrease of melanoma tumor growth in mGlu1 receptor-expressing melanomas. Here, we demonstrate that mGlu1 receptors, which have been previously characterized as oncogenes, also behave like dependence receptors by creating a dependence on glutamate for sustained cell viability. In the mGlu1 receptor-expressing melanoma cell lines, SK-MEL-2 (SK2) and SK-MEL-5 (SK5), we show that glutamate is both necessary and sufficient to maintain cell viability, regardless of underlying genetic mutations. Addition of glutamate increased DNA synthesis while removal of glutamate not only suppressed DNA synthesis, but also promoted cell death in SK2 and SK5 melanoma cells. Using genetic and pharmacological inhibitors we established that this effect of glutamate is mediated by activation of mGlu1 receptors. The stimulatory potential of mGlu1 receptors was further confirmed in vivo in a melanoma cell xenograft model. In this model, subcutaneous injection of SK5 cells with shRNA targeted downregulation of mGlu1 receptors resulted in a decrease in the rate of tumor growth relative to control. We also demonstrate for the first time, that a selective mGlu1 receptor antagonist, JNJ16259685 [(3,4-Dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone], slows SK2 and SK5 melanoma tumor growth in vivo. Taken together, these data suggest that pharmacological inhibition of mGlu1 receptors may be a novel approach for the treatment of metastatic melanoma.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
COT/MAP3K8 drives resistance to RAF inhibition through MAP kinase pathway reactivation
- Record: found
- Abstract: found
- Article: not found
Pharmacology and functions of metabotropic glutamate receptors.
- Record: found
- Abstract: found
- Article: not found
